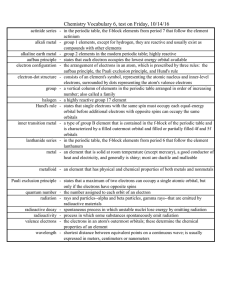
alkaline earth metals
... The periodic table has distinct regions. • Position of an element on the PT reveals something about how reactive the element is. • Elements in Groups 1 and 17 are especially reactive. • Group 18 (the Noble Gases) are least reactive. ...
... The periodic table has distinct regions. • Position of an element on the PT reveals something about how reactive the element is. • Elements in Groups 1 and 17 are especially reactive. • Group 18 (the Noble Gases) are least reactive. ...
Notes
... electron configuration, but understandable in terms of the additional stability a half filled or completely filled sub-shell possesses. In periods 5 and 6 the electron configuration of the transition metals appears more irregular and I do not expect you to predict them! ...
... electron configuration, but understandable in terms of the additional stability a half filled or completely filled sub-shell possesses. In periods 5 and 6 the electron configuration of the transition metals appears more irregular and I do not expect you to predict them! ...
PERIODIC TABLE - WordPress.com
... 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does iron belong to in the Periodic Table? 8. Which ...
... 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does iron belong to in the Periodic Table? 8. Which ...
Grouping of Elements in the Periodic Table
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
Section 15.2 - CPO Science
... 15.2 Metals and metal alloys Titanium combines the strength and hardness of steel with the light weight of aluminum. Titanium, a rare and expensive alloy, is used for military aircraft and racing bicycles. ...
... 15.2 Metals and metal alloys Titanium combines the strength and hardness of steel with the light weight of aluminum. Titanium, a rare and expensive alloy, is used for military aircraft and racing bicycles. ...
atomic number
... element has. For instance, hydrogen has 1 proton, so it’s atomic number is 1. The atomic number is unique to that element. No two elements have the same atomic number. ...
... element has. For instance, hydrogen has 1 proton, so it’s atomic number is 1. The atomic number is unique to that element. No two elements have the same atomic number. ...
The Chinese High School
... What is steel? It cannot be found in the periodic table – so it is not an element! On the same note, what is brass? Or bronze? What is 12K/18K/24K gold? What is the difference between the different types of gold? All these questions can be answered with one key word – do you know what it is? ...
... What is steel? It cannot be found in the periodic table – so it is not an element! On the same note, what is brass? Or bronze? What is 12K/18K/24K gold? What is the difference between the different types of gold? All these questions can be answered with one key word – do you know what it is? ...
CI_Chap_1_Test_A_Study_Guide
... The most common element in the universe is hydrogen. Atoms of an element always have a certain number of protons. Isotopes of an element differ in the number of neutrons. When an atom loses one or more electrons in becomes an ion with a + charge. The atomic mass number is the total number of protons ...
... The most common element in the universe is hydrogen. Atoms of an element always have a certain number of protons. Isotopes of an element differ in the number of neutrons. When an atom loses one or more electrons in becomes an ion with a + charge. The atomic mass number is the total number of protons ...
Chapter 8 Study Guide
... Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- most reactive non-metals; “salt formers” Noble Gases (VIII)- colorless, odorless gases; unreactive because they have a full outer shell of electrons Rare-Earth Elements- at bottom of peri ...
... Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- most reactive non-metals; “salt formers” Noble Gases (VIII)- colorless, odorless gases; unreactive because they have a full outer shell of electrons Rare-Earth Elements- at bottom of peri ...
Section 12.4 - CPO Science
... 12.4 Metals and metal alloys Titanium combines the strength and hardness of steel with the light weight of aluminum. Titanium, a rare and expensive alloy, is used for military aircraft and racing bicycles. ...
... 12.4 Metals and metal alloys Titanium combines the strength and hardness of steel with the light weight of aluminum. Titanium, a rare and expensive alloy, is used for military aircraft and racing bicycles. ...
Lab 18
... conduct heat and electricity, Malleable and ductile (flexible) as solids. Also, generally the melting points of non-metals are generally lower than metals. And finally compounds of metals with non-metals tend to be ionic in nature as opposed to non-metal’s being nonmetal oxides are acidic oxide. 4. ...
... conduct heat and electricity, Malleable and ductile (flexible) as solids. Also, generally the melting points of non-metals are generally lower than metals. And finally compounds of metals with non-metals tend to be ionic in nature as opposed to non-metal’s being nonmetal oxides are acidic oxide. 4. ...
Notes 3-2
... material that can be pulled out into a long wire. Conductor – a substance that transmits heat or electricity easily. Magnetic – a characteristic of metals in which it is attracted to magnets or can be made into a magnet. Chemical Properties of Metals Reactivity – ease and speed with which an element ...
... material that can be pulled out into a long wire. Conductor – a substance that transmits heat or electricity easily. Magnetic – a characteristic of metals in which it is attracted to magnets or can be made into a magnet. Chemical Properties of Metals Reactivity – ease and speed with which an element ...
UNIT 3 –TEST REVIEW 1 Atoms of which of the
... Zinc IS IN SAME GROUP AS Cd F gold (Au) G zinc (Zn) H silver (Ag) J copper (Cu) ...
... Zinc IS IN SAME GROUP AS Cd F gold (Au) G zinc (Zn) H silver (Ag) J copper (Cu) ...
In the space provided, write the letter of the term or phrase that best
... ______ 7. In nature the alkali metals are found only in compounds because they a. have small atoms. b. are very reactive elements. c. are rare elements. d. each have a stable octet. ______ 8. To which group does hydrogen belong? a. Group 1 b. Group 2 ...
... ______ 7. In nature the alkali metals are found only in compounds because they a. have small atoms. b. are very reactive elements. c. are rare elements. d. each have a stable octet. ______ 8. To which group does hydrogen belong? a. Group 1 b. Group 2 ...
Atomic and Molecular Structure – Standard 1 Review
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
- Priddy ISD
... alkali metal - group 1 elements, except for hydrogen, they are reactive and usually exist as compounds with other elements alkaline earth metal - group 2 elements in the modern periodic table; highly reactive aufbau principle - states that each electron occupies the lowest energy orbital available e ...
... alkali metal - group 1 elements, except for hydrogen, they are reactive and usually exist as compounds with other elements alkaline earth metal - group 2 elements in the modern periodic table; highly reactive aufbau principle - states that each electron occupies the lowest energy orbital available e ...
SOL Review Station: Equipment, Accuracy, Precision and Lab Safety
... 1. What is formed if you change the following in an atom? a. Protons ...
... 1. What is formed if you change the following in an atom? a. Protons ...
Section 14.2 - CPO Science
... • Because they are so different from metals, elements on the far right of the table are called non-metals. • Nonmetals make good insulators. • An insulator is a material which slows down or stops the flow of either heat or electricity. ...
... • Because they are so different from metals, elements on the far right of the table are called non-metals. • Nonmetals make good insulators. • An insulator is a material which slows down or stops the flow of either heat or electricity. ...
Basics of the Periodic Table
... 1. __________________________________ is the only nonmetal on the left side of the Periodic Table. 2. A row of elements is called a ________________________________________. 3. A column of elements is called a _________________________________________ or _____________________________________________ ...
... 1. __________________________________ is the only nonmetal on the left side of the Periodic Table. 2. A row of elements is called a ________________________________________. 3. A column of elements is called a _________________________________________ or _____________________________________________ ...
9The-Periodic-table1 (3).pptx
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
Name: Per: _____ Date: ______ Unit 5 Redemption Packet: The
... 15. Which noble gas does not have 8 valence electrons? _______________ 16. How is the atomic radius of an atom determined? _________________________________ ________________________________________________________________________ 17. a) How does atomic radius change across a period? _______________ ...
... 15. Which noble gas does not have 8 valence electrons? _______________ 16. How is the atomic radius of an atom determined? _________________________________ ________________________________________________________________________ 17. a) How does atomic radius change across a period? _______________ ...
Unit 4 Review - Davis
... The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasi ...
... The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasi ...