The Periodic Table
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
Name: Date: Period: ______ Graphing Periodic Trends Purpose:To
... Jj is considered to be the “last” (highest atomic number) naturally-occurring element; it forms beautiful crystals and is used in that pink stomach-ache medicine that makes your tongue turn black. ...
... Jj is considered to be the “last” (highest atomic number) naturally-occurring element; it forms beautiful crystals and is used in that pink stomach-ache medicine that makes your tongue turn black. ...
Chapter 5 Section 1 - Ms. Halbohm`s Classroom
... 3. What is the relationship between the electron configuration of an element and the period in which that element appears in the periodic table? 4. What information is provided by the specific block location of an element? Identify, by number, the groups located within each of the four block areas. ...
... 3. What is the relationship between the electron configuration of an element and the period in which that element appears in the periodic table? 4. What information is provided by the specific block location of an element? Identify, by number, the groups located within each of the four block areas. ...
Word - The Chemistry Book
... substances: fire, earth, water, and air. b. Alchemists discovered mercury, sulfur, and antimony. c. Robert Boyle insisted science should be grounded in experiments; defined an element as a substance that could not be broken down. 3.1 The Elements 1. 88 elements occur naturally; other elements are ma ...
... substances: fire, earth, water, and air. b. Alchemists discovered mercury, sulfur, and antimony. c. Robert Boyle insisted science should be grounded in experiments; defined an element as a substance that could not be broken down. 3.1 The Elements 1. 88 elements occur naturally; other elements are ma ...
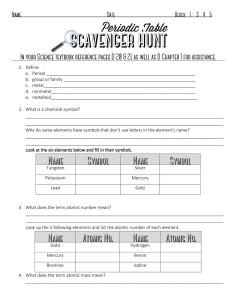
SCH3U Periodic Table Worksheet 1. Where are the most active
... 3. As you go from left to right across a period, the atomic radius (increases/decreases). Why? Decreases. More positively charged protons in the nucleus pulling electrons in closer. 4. As you travel down a group, the atomic radius (increases/decreases). Why? Increases. Adding extra shells – the inne ...
... 3. As you go from left to right across a period, the atomic radius (increases/decreases). Why? Decreases. More positively charged protons in the nucleus pulling electrons in closer. 4. As you travel down a group, the atomic radius (increases/decreases). Why? Increases. Adding extra shells – the inne ...
Matter: Building Blocks of the Universe Chapter 5 Classification of
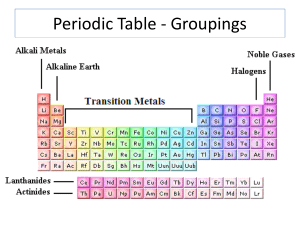
... the same number of valence electrons Family 1—Alkali—soft, silver, white, shiny—react or combine with other elements easily—never found alone in nature Family 2—Alkaline—Earth metals—very reactive Between Family 2 and 13 are the transition metals—these are the metals you are most familiar with ...
... the same number of valence electrons Family 1—Alkali—soft, silver, white, shiny—react or combine with other elements easily—never found alone in nature Family 2—Alkaline—Earth metals—very reactive Between Family 2 and 13 are the transition metals—these are the metals you are most familiar with ...
C3 The Periodic Table
... • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • He left GAPS because he predicted new elements would fit in, as they were discovered. He was right! ...
... • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • He left GAPS because he predicted new elements would fit in, as they were discovered. He was right! ...
Who`s in this family?
... • It is used to build some airplanes • It activates many of the enzymes that speed up processes in the human body • It combines with many other elements to form useful compounds such as, milk of magnesia & Epsom salts ...
... • It is used to build some airplanes • It activates many of the enzymes that speed up processes in the human body • It combines with many other elements to form useful compounds such as, milk of magnesia & Epsom salts ...
The Periodic Table - Miss Schaefer`s Science Grade 8
... • Elements on the periodic table can be grouped into families (or groups) based on their chemical properties. – We call them “families” because the elements in each family are “related.” ...
... • Elements on the periodic table can be grouped into families (or groups) based on their chemical properties. – We call them “families” because the elements in each family are “related.” ...
Periodic Table cloze activity.
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
File
... 18. The elements on the periodic table are arranged by increasing _____________ ________________. (hint: what number continually gets bigger as the table proceeds?) 19. Define what a group is on the periodic table. 20. Define what a period is on the periodic table. 21. Moving from left to right acro ...
... 18. The elements on the periodic table are arranged by increasing _____________ ________________. (hint: what number continually gets bigger as the table proceeds?) 19. Define what a group is on the periodic table. 20. Define what a period is on the periodic table. 21. Moving from left to right acro ...
1. Define: a. Period b. group or family
... Define transition metals Where are they on the Periodic table? List 3 examples: 10. What are most elements: metals, non-metals or metalloids?_______________________________ 11. Complete the chart below: ...
... Define transition metals Where are they on the Periodic table? List 3 examples: 10. What are most elements: metals, non-metals or metalloids?_______________________________ 11. Complete the chart below: ...
The Periodic Table notes
... noble gas family. These elements do not interact with other elements and they prefer to be alone. They all have 8 valence electrons. ...
... noble gas family. These elements do not interact with other elements and they prefer to be alone. They all have 8 valence electrons. ...
Main Group and Transition Elements (15 h)
... forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions. ...
... forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions. ...
Topics 3 and 13 Outline
... elements Zn, Cr and Cu do not follow these patterns and are therefore considered anomalous in the first-row d-block. (3.1) Understandings: • Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and display catalytic and magnetic properties. • Z ...
... elements Zn, Cr and Cu do not follow these patterns and are therefore considered anomalous in the first-row d-block. (3.1) Understandings: • Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and display catalytic and magnetic properties. • Z ...
File
... Atoms of different elements have different numbers of protons that correspond to their atomic number ...
... Atoms of different elements have different numbers of protons that correspond to their atomic number ...
Science Review Sheet: Periodic Table Test Name: ______ Study
... Study periodic table notes. Know the properties of Alkali Metals, Alkaline Earth Metals, Halides/Halogens and Noble Gases. Know how to calculate atomic mass, # of protons, # of electrons, and #of neutrons in an atom. 1. What are the three subatomic particles? Where are they found within an atom? Wha ...
... Study periodic table notes. Know the properties of Alkali Metals, Alkaline Earth Metals, Halides/Halogens and Noble Gases. Know how to calculate atomic mass, # of protons, # of electrons, and #of neutrons in an atom. 1. What are the three subatomic particles? Where are they found within an atom? Wha ...
The Periodic Table - Lincoln Park High School
... • Families may be one column, or several columns put together. • Families have names rather than numbers. ...
... • Families may be one column, or several columns put together. • Families have names rather than numbers. ...
Periodic trends Tempura
... When Mendeleev was arranging the periodic table, many elements had not been discovered. Mendeleev left gaps for these missing elements and predicted many of their properties from their location on the periodic table. 4. List some properties of metals and nonmetals. Metals- shiny, conduct heat and el ...
... When Mendeleev was arranging the periodic table, many elements had not been discovered. Mendeleev left gaps for these missing elements and predicted many of their properties from their location on the periodic table. 4. List some properties of metals and nonmetals. Metals- shiny, conduct heat and el ...
Elements and Their Properties
... extremely rare and radioactive. A radioactive element is one in which the nucleus breaks down and gives off particles and energy. ...
... extremely rare and radioactive. A radioactive element is one in which the nucleus breaks down and gives off particles and energy. ...
Periodic Table
... fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the fewer electrons needed to fill the valance are very reactive and combine easily to form compounds. Atoms with full valances do not react to form compoun ...
... fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the fewer electrons needed to fill the valance are very reactive and combine easily to form compounds. Atoms with full valances do not react to form compoun ...
Chapter 6 Periodic law- states that when the elements are arranged
... periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated ...
... periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated ...