The Periodic Table - Ms. Dormer
... First periodic table 63 known elements at the time Mendeleev’s table contains gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
... First periodic table 63 known elements at the time Mendeleev’s table contains gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
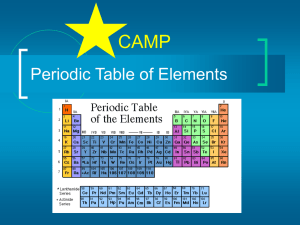
Periodic Table of Elements
... electrons in their outer energy level. • This means that they tend to accept electrons when they combine chemically. • Those with 8 valence electrons are stable and non-reactive. ...
... electrons in their outer energy level. • This means that they tend to accept electrons when they combine chemically. • Those with 8 valence electrons are stable and non-reactive. ...
Chemistry Study Cards Chapter 5 (3-2) The length of each period in
... Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? ...
... Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? ...
The Periodic Table Worksheet
... 11. There are two rows of elements on the bottom of the table. These elements are the rare earth metals. What is the name given to each of these rows of elements? ___________________ ___________________ 12. Name two elements in group VIIA. ___________________ ___________________ ...
... 11. There are two rows of elements on the bottom of the table. These elements are the rare earth metals. What is the name given to each of these rows of elements? ___________________ ___________________ 12. Name two elements in group VIIA. ___________________ ___________________ ...
Periodic Table2011
... • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) – Atoms bond until this level ...
... • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) – Atoms bond until this level ...
File
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
The Periodic Table PP
... • A vertical column of the periodic table is called a group • Elements in a group share similar chemical ...
... • A vertical column of the periodic table is called a group • Elements in a group share similar chemical ...
Oxygen - Hingham
... Five electrons in the outermost energy level. As (5 Valence Electrons) Sb They physical and chemical properties that are strikingly Bi different. N and P make up fertilizer. ...
... Five electrons in the outermost energy level. As (5 Valence Electrons) Sb They physical and chemical properties that are strikingly Bi different. N and P make up fertilizer. ...
Chemical-Periodicity
... Outermost s sublevel and the nearby d sublevel contains electrons Called the group B elements ...
... Outermost s sublevel and the nearby d sublevel contains electrons Called the group B elements ...
Test Review
... _____ solids with high _____ melting points. d.Transition metal unpaired d-electrons have the ability to move into the s __ level. Because of this, many transition metals can form several different charged ions. e. Transition metals contain the __________ preciousmetals (like gold & silver) ...
... _____ solids with high _____ melting points. d.Transition metal unpaired d-electrons have the ability to move into the s __ level. Because of this, many transition metals can form several different charged ions. e. Transition metals contain the __________ preciousmetals (like gold & silver) ...
3.08_Periodic Table and the Atom
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
20151023082664
... How are sounds of musical notes related to the periodic table How is the modern periodic table arranged How are elements related in groups Why is the gram measurement unit not useful for element mass What is the convenient way to compare masses of atoms Know the different ways to classify ele ...
... How are sounds of musical notes related to the periodic table How is the modern periodic table arranged How are elements related in groups Why is the gram measurement unit not useful for element mass What is the convenient way to compare masses of atoms Know the different ways to classify ele ...
The Atom and how it is organized - Cashmere
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
Periodic Table
... Electronegativity is the ability of a nucleus to attract its valence/bonding electrons. It follows certain trends on the table: As you go across (left to right) it gets stronger ...
... Electronegativity is the ability of a nucleus to attract its valence/bonding electrons. It follows certain trends on the table: As you go across (left to right) it gets stronger ...
atomic number - Net Start Class
... Metalloids (metal-like) have properties of both metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and malleable. ...
... Metalloids (metal-like) have properties of both metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and malleable. ...
Powerpoint - Valence Electrons
... Br is a liquid and I is a solid F is the most reactive and I is the least Have a -1 ion charge ...
... Br is a liquid and I is a solid F is the most reactive and I is the least Have a -1 ion charge ...
Periodic Table Cloze - Science
... composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
... composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
File
... Elements are listed horizontally according to their atomic number - number of protons Periods are the horizontal rows of elements Halogens - highly reactive, Elements are listed vertically according to their chemical properties gases except Br liquid Groups or Families are arranged vertically Noble ...
... Elements are listed horizontally according to their atomic number - number of protons Periods are the horizontal rows of elements Halogens - highly reactive, Elements are listed vertically according to their chemical properties gases except Br liquid Groups or Families are arranged vertically Noble ...
Chapter 5
... Group 3-12: Transition metals Electron configurations end in d1 for group 3 and end in d10 for group 12 Good conductors of heat and electricity Less reactive than s block metals Some exist in nature as free elements ...
... Group 3-12: Transition metals Electron configurations end in d1 for group 3 and end in d10 for group 12 Good conductors of heat and electricity Less reactive than s block metals Some exist in nature as free elements ...
The Periodic Table Memorize which elements are gases and
... E. Some elements exist as two or more forms in the same phase called allotropes which have different properties; include O and C F. Chemistry of selected elements 1. Alkali metals – Group 1 elements; have silvery appearance; are soft; have low melting points; are too reactive to exist in nature as f ...
... E. Some elements exist as two or more forms in the same phase called allotropes which have different properties; include O and C F. Chemistry of selected elements 1. Alkali metals – Group 1 elements; have silvery appearance; are soft; have low melting points; are too reactive to exist in nature as f ...
The Periodic Table
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
Page|1 - askIITians
... Q26. What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group? ...
... Q26. What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group? ...
PPT Periodic Families from Class
... after the element that makes up 78% of our atmosphere. • This family includes non-metals, metalloids, and metals. • Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when ...
... after the element that makes up 78% of our atmosphere. • This family includes non-metals, metalloids, and metals. • Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when ...
Chapter 6 Study Guide
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...