atomic number
... 3- The strongest non-metallic element lies in group ----------------. ( 7A) 4- ------------------- lie preceding noble gases in the periodic table, and during the chemical reaction they form -------------------ions. ( non metals - -ve ions ) ...
... 3- The strongest non-metallic element lies in group ----------------. ( 7A) 4- ------------------- lie preceding noble gases in the periodic table, and during the chemical reaction they form -------------------ions. ( non metals - -ve ions ) ...
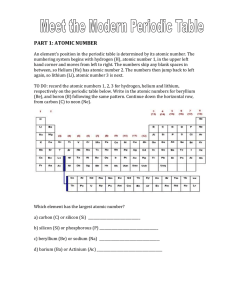
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
Section 15.1
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
Elements and the Periodic Table Practice Test
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... Solid carbon is added to aluminum oxide in order to form pure aluminum metal and carbon dioxide gas. ...
... Solid carbon is added to aluminum oxide in order to form pure aluminum metal and carbon dioxide gas. ...
2 - CronScience
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
SAT Practice Test 3
... HCl is a proton donor Powdered zinc has a greater surface area NH3 is a polar substance Water boils when the vapor pressure of the water is equal to the atmospheric pressure In an exothermic reaction the products have less potential energy than the reactants Pressure and volume have a direct relatio ...
... HCl is a proton donor Powdered zinc has a greater surface area NH3 is a polar substance Water boils when the vapor pressure of the water is equal to the atmospheric pressure In an exothermic reaction the products have less potential energy than the reactants Pressure and volume have a direct relatio ...
Preview Sample 1
... Structure of the Atom Use the structure of atoms to describe how the composition of atoms can differ. Understand the experiments used to determine the structure of the atom. Compare the properties of protons, electrons, and neutrons. Determine the number of protons, electrons, and neutrons f ...
... Structure of the Atom Use the structure of atoms to describe how the composition of atoms can differ. Understand the experiments used to determine the structure of the atom. Compare the properties of protons, electrons, and neutrons. Determine the number of protons, electrons, and neutrons f ...
1 - Montville.net
... Assuming the process is 95.0% efficient, how many grams of CaSO4 may be produced from 1.00 x 102 grams of SO2? 202 g ...
... Assuming the process is 95.0% efficient, how many grams of CaSO4 may be produced from 1.00 x 102 grams of SO2? 202 g ...
Acids and Bases and Aqueous Equilibria
... Gilbert Newton Lewis (1875-1946) In a 1923 paper, Lewis wrote: "We are so habituated to the use of water as a solvent, and our data are so frequently limited to those obtained in aqueous solutions, that we frequently define an acid or a base as a substance whose aqueous solution gives, respectively, ...
... Gilbert Newton Lewis (1875-1946) In a 1923 paper, Lewis wrote: "We are so habituated to the use of water as a solvent, and our data are so frequently limited to those obtained in aqueous solutions, that we frequently define an acid or a base as a substance whose aqueous solution gives, respectively, ...
Name Period
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
Chapters 6, 8
... There are ~ 11 million chemical compounds. Some of the arbitrary (common) names you may recognize (water = hydrogen oxide H2O, laughing gas = dinitrogen monoxide N2O, quicksilver = mercury Hg). One cannot memorize arbitrary names of all of them. Chemical nomenclature is the system of names for compo ...
... There are ~ 11 million chemical compounds. Some of the arbitrary (common) names you may recognize (water = hydrogen oxide H2O, laughing gas = dinitrogen monoxide N2O, quicksilver = mercury Hg). One cannot memorize arbitrary names of all of them. Chemical nomenclature is the system of names for compo ...
Study Guide – Honors Chemistry: Exam One
... Based on ionic charge, be able to predict the ratio with which anions and cations will combine to form a compound. Predict the formula for the following ionic compounds (use your book to remind yourself of some of the polyatomic ions – you will be given a list of names and formulas for polyatomic io ...
... Based on ionic charge, be able to predict the ratio with which anions and cations will combine to form a compound. Predict the formula for the following ionic compounds (use your book to remind yourself of some of the polyatomic ions – you will be given a list of names and formulas for polyatomic io ...
Name
... identify substances and determine the number of atoms of each element. Ⓡ 8.5 (E) Chemical Reactions: Students will be able to investigate how evidences of chemical reactions indicate that new substances are formed. Ⓡ 8.5 (F) Balancing Equations: Students will be able to recognize whether or not a ch ...
... identify substances and determine the number of atoms of each element. Ⓡ 8.5 (E) Chemical Reactions: Students will be able to investigate how evidences of chemical reactions indicate that new substances are formed. Ⓡ 8.5 (F) Balancing Equations: Students will be able to recognize whether or not a ch ...
Give the name and symbol for the element found in
... that these blank spaces represented elements that had not yet been discovered, and based on their position in the periodic table, Mendeleev was able to predict the properties of these undiscovered elements. When these elements were discovered, their properties were very similar to those predicted by ...
... that these blank spaces represented elements that had not yet been discovered, and based on their position in the periodic table, Mendeleev was able to predict the properties of these undiscovered elements. When these elements were discovered, their properties were very similar to those predicted by ...
2016 - Specimen Paper 2 - Cambridge International Examinations
... reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. Cambridge International Examinations is part of the Cambridge A ...
... reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. Cambridge International Examinations is part of the Cambridge A ...
Chapter 2 - Test Bank
... called inert gases, in Group 8A). Another group that is useful to recognize is the three metals: copper (Cu), silver (Ag), and gold (Au), which make up the “coinage metals.” This group will be important when we discuss exceptions to the electron configurations of these elements in a later chapter. S ...
... called inert gases, in Group 8A). Another group that is useful to recognize is the three metals: copper (Cu), silver (Ag), and gold (Au), which make up the “coinage metals.” This group will be important when we discuss exceptions to the electron configurations of these elements in a later chapter. S ...
Power point types of chemical rxn
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
Document
... Complete the table that shows the reaction, if any, of the oxides with acid and alkali. Indicate a reaction with "R" and no reaction with "NR". ...
... Complete the table that shows the reaction, if any, of the oxides with acid and alkali. Indicate a reaction with "R" and no reaction with "NR". ...
AQA C2 revision book
... held together by strong forces called covalent bonds, but there are only very weak forces between the molecules. This means: 1) They have low melting and boiling points (many are liquids or gases). 2) They tend to be soft and/or have little strength. 3) They do not conduct electricity Simple molecul ...
... held together by strong forces called covalent bonds, but there are only very weak forces between the molecules. This means: 1) They have low melting and boiling points (many are liquids or gases). 2) They tend to be soft and/or have little strength. 3) They do not conduct electricity Simple molecul ...
FREE Sample Here
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
Periodic Trends - Naperville Community Unit School
... masses, some of the inconsistencies associated with Mendeleev's table were eliminated. The modern periodic table is based on Moseley's Periodic Law (atomic numbers). ...
... masses, some of the inconsistencies associated with Mendeleev's table were eliminated. The modern periodic table is based on Moseley's Periodic Law (atomic numbers). ...