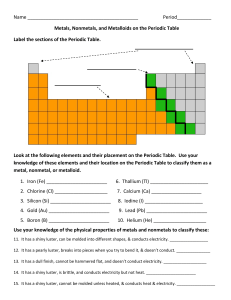
Name Period_____________ Metals, Nonmetals, and Metalloids on
... Look at the following elements and their placement on the Periodic Table. Use your knowledge of these elements and their location on the Periodic Table to classify them as a metal, nonmetal, or metalloid. 1. Iron (Fe) _______________________ ...
... Look at the following elements and their placement on the Periodic Table. Use your knowledge of these elements and their location on the Periodic Table to classify them as a metal, nonmetal, or metalloid. 1. Iron (Fe) _______________________ ...
CHAPTER 5 - THE PERIODIC LAW
... - sufficiently reactive (excluding bismuth) - found in nature only in the form of ___________________ - HALOGENS - ____________________________________ - most _____________________nonmetals (reason is they only have 7 electrons in outermost energy level - react vigorously with most metals to form ex ...
... - sufficiently reactive (excluding bismuth) - found in nature only in the form of ___________________ - HALOGENS - ____________________________________ - most _____________________nonmetals (reason is they only have 7 electrons in outermost energy level - react vigorously with most metals to form ex ...
Chapter 11 Chemical Reactions
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
www.xtremepapers.net
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
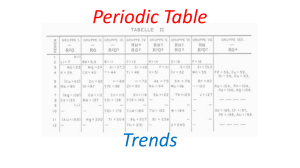
2- Periodic Trends
... •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
... •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
assignment-07-a3
... 20- Which of the following has the highest ionization energy? Na, Na+, K+, Mg2+? a) Na+ b) Mg2+ c) K+ d) Na (This magnesium ion has the largest positive charge of the list of atoms and ions, and has an inertgas-like electron configuration.) 21- Which of the following are metalloids? a) O, S, Se b) A ...
... 20- Which of the following has the highest ionization energy? Na, Na+, K+, Mg2+? a) Na+ b) Mg2+ c) K+ d) Na (This magnesium ion has the largest positive charge of the list of atoms and ions, and has an inertgas-like electron configuration.) 21- Which of the following are metalloids? a) O, S, Se b) A ...
Slide 1
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
Exam Review
... 21. Compared to the stability of the original atom, the stability of its ion that resembles a noble gas configuration would be a) identical b) sometimes less c) less d) greater 22. The formation of bonds between atoms depends on __. a) the electron configurations of the atoms involved c) both of the ...
... 21. Compared to the stability of the original atom, the stability of its ion that resembles a noble gas configuration would be a) identical b) sometimes less c) less d) greater 22. The formation of bonds between atoms depends on __. a) the electron configurations of the atoms involved c) both of the ...
Test Review
... e. Exist in all 3 _______ states of matter (s, l, g) metals especially alkali, to produce salts. f. React readily with ________, (halogen = salt former) ...
... e. Exist in all 3 _______ states of matter (s, l, g) metals especially alkali, to produce salts. f. React readily with ________, (halogen = salt former) ...
Chem MCQ for Class-9th
... 13. Triple covalent bond involves how many electrons? a. Eight b. six c. four d.only three ...
... 13. Triple covalent bond involves how many electrons? a. Eight b. six c. four d.only three ...
The Packet
... a.________________A compound with the molecular formula C6H6 has the same simplest formula. b.________________The mass percent of copper in CuO is less than in Cu2O. c.________________The limiting reactant is the one present in the smallest number of grams. d.________________Since C3H6O3 and C6H12O6 ...
... a.________________A compound with the molecular formula C6H6 has the same simplest formula. b.________________The mass percent of copper in CuO is less than in Cu2O. c.________________The limiting reactant is the one present in the smallest number of grams. d.________________Since C3H6O3 and C6H12O6 ...
Chemicals: What`s in? What`s out?
... should be removed due to their hazardous nature. Remember that any chemical can be considered hazardous if not used appropriately. Again, consult the MSDS for additional information. Ammonium dichromate—(NH4) 2Cr 2 O 7 (toxic byproducts) Benzene (carcinogen) Calcium carbide—CaC2 (explosion hazard) C ...
... should be removed due to their hazardous nature. Remember that any chemical can be considered hazardous if not used appropriately. Again, consult the MSDS for additional information. Ammonium dichromate—(NH4) 2Cr 2 O 7 (toxic byproducts) Benzene (carcinogen) Calcium carbide—CaC2 (explosion hazard) C ...
Atom/Elements Study Guide
... The atom is the smallest particle that cannot be broken down by ordinary means; it is the building block of all matter. A neutral atom is one that carries no charge because the protons (+) and electrons (-) are equal, therefore balancing each other out. Elements are the simple building blocks of all ...
... The atom is the smallest particle that cannot be broken down by ordinary means; it is the building block of all matter. A neutral atom is one that carries no charge because the protons (+) and electrons (-) are equal, therefore balancing each other out. Elements are the simple building blocks of all ...
Document
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
Summer Assignment for AP Chemistry: I hope you are all ready for a
... double replacement reactions and the activity series for single replacement reactions. Hint: when writing these reactions, ignore all of the information about heat, or bubbling, or mixing. These are just excess words used to make complete sentences. Simply pull out the chemical formulas. For example ...
... double replacement reactions and the activity series for single replacement reactions. Hint: when writing these reactions, ignore all of the information about heat, or bubbling, or mixing. These are just excess words used to make complete sentences. Simply pull out the chemical formulas. For example ...
9The-Periodic-table1 (3).pptx
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
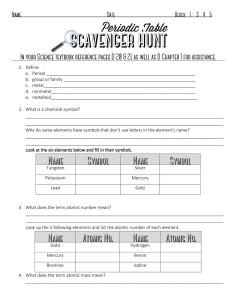
1. Define: a. Period b. group or family
... reactivity of the elements as you go left to right across the periodic table. Describe what happens to atomic mass as you go left to right across the periodic table. Describe what happens to atomic mass as you from top to bottom on a periodic table Describe what happens to atom size as you go from t ...
... reactivity of the elements as you go left to right across the periodic table. Describe what happens to atomic mass as you go left to right across the periodic table. Describe what happens to atomic mass as you from top to bottom on a periodic table Describe what happens to atom size as you go from t ...
AP Chemistry Summer Assignment
... f. Krypton g. Fluorine h. Scandium I. Arsenic J. Potassium K. Sodium l. chloride m. Iron n. Zinc o. tin 10. Write the latin names for each of the elements symbols: a. Na b. Au c. Ag d. Sn e. Fe f. Hg ...
... f. Krypton g. Fluorine h. Scandium I. Arsenic J. Potassium K. Sodium l. chloride m. Iron n. Zinc o. tin 10. Write the latin names for each of the elements symbols: a. Na b. Au c. Ag d. Sn e. Fe f. Hg ...
Name: Homeroom
... Atoms that have the same number of protons but different numbers of neutrons are called isotopes. An isotope can be identified by using a value called an atomic mass. Atomic mass is the sum of the number of neutrons and the number of protons in the nucleus. ...
... Atoms that have the same number of protons but different numbers of neutrons are called isotopes. An isotope can be identified by using a value called an atomic mass. Atomic mass is the sum of the number of neutrons and the number of protons in the nucleus. ...
practice-exam-2
... 24. [7 points] What of the following is the correct balanced net ionic equation for the reaction between silver hydroxide and hydrochloric acid? (a) AgOH (s) + HCl (aq) � H2O (l) + AgCl (s) (b) AgOH (s) + H+ (aq) � H2O (l) + Ag+ (aq) ...
... 24. [7 points] What of the following is the correct balanced net ionic equation for the reaction between silver hydroxide and hydrochloric acid? (a) AgOH (s) + HCl (aq) � H2O (l) + AgCl (s) (b) AgOH (s) + H+ (aq) � H2O (l) + Ag+ (aq) ...
Chem 30A Fa_06 FE Review
... (d) Iron-55 ( 26 Fe) decays by electron capture (e) Thorium-230 (230Th) decays by alpha emission ...
... (d) Iron-55 ( 26 Fe) decays by electron capture (e) Thorium-230 (230Th) decays by alpha emission ...
Name
... All atoms of one element have the same number of _________________, but do not all have the same mass. Some are heavier because they have a different number of ______________________ in their nuclei. Atoms that have the _____________ number of protons but ______________ numbers of neutrons are calle ...
... All atoms of one element have the same number of _________________, but do not all have the same mass. Some are heavier because they have a different number of ______________________ in their nuclei. Atoms that have the _____________ number of protons but ______________ numbers of neutrons are calle ...
Preview Sample 1
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
Test 1. 2nd prep. ques
... Write balanced chemical equation which expresses each of the following reaction 1- Bromine with potassium iodine ----Br2+2 KI ⤍2KBr + I2------------------------------------------------------------------2- Sodium with water ------2Na + 2H2O⇢ 2 NaOH + H2------------------------------------------------ ...
... Write balanced chemical equation which expresses each of the following reaction 1- Bromine with potassium iodine ----Br2+2 KI ⤍2KBr + I2------------------------------------------------------------------2- Sodium with water ------2Na + 2H2O⇢ 2 NaOH + H2------------------------------------------------ ...