Set #4 - comsics
... Hydrogen atoms in states of high quantum number have been created in the laboratory and observed in space. (a) Find the quantum number of the Bohr orbit in a hydrogen atom whose radius is 0.0199 mm. (b) What is the energy of a hydrogen atom in this case? ...
... Hydrogen atoms in states of high quantum number have been created in the laboratory and observed in space. (a) Find the quantum number of the Bohr orbit in a hydrogen atom whose radius is 0.0199 mm. (b) What is the energy of a hydrogen atom in this case? ...
03-02BohrAtom
... eV to -13.6 eV, so it releases 10.2 eV of energy. A photon with this energy has this wavelength: ...
... eV to -13.6 eV, so it releases 10.2 eV of energy. A photon with this energy has this wavelength: ...
Objective A - TuHS Physics Homepage
... 2. Write a formula for the line using the concept behind the photo-electric effect. 3. What is the slope of the line? 4. What is the meaning of the x-intercept? 5. How does the graph support photon theory over wave theory? Objective H: Matter Waves p = h/, p = mv Problems: Chapter 27: 14(1.1E-27 kg ...
... 2. Write a formula for the line using the concept behind the photo-electric effect. 3. What is the slope of the line? 4. What is the meaning of the x-intercept? 5. How does the graph support photon theory over wave theory? Objective H: Matter Waves p = h/, p = mv Problems: Chapter 27: 14(1.1E-27 kg ...
Early Quantum Theory and Models of the Atom
... • Bohr studied Rutherford’s planetary model and found it had validity • But to make it work the newly developing quantum theory would have to be incorporated • Plank and Einstein had shown that in heated solids, the energy of oscillating electric charges must change from one discrete energy state to ...
... • Bohr studied Rutherford’s planetary model and found it had validity • But to make it work the newly developing quantum theory would have to be incorporated • Plank and Einstein had shown that in heated solids, the energy of oscillating electric charges must change from one discrete energy state to ...
do physics online from quanta to quarks the bohr model of the atom
... Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance. However, the Bohr models were an important step in the development of quantum mechanics. Quantum mechanics is a mathematical theory to account for the atomic related behaviour of our ph ...
... Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance. However, the Bohr models were an important step in the development of quantum mechanics. Quantum mechanics is a mathematical theory to account for the atomic related behaviour of our ph ...
Chapter 5 Sec. 2 Bohr`s Model and the Quantum Mechanical Model
... He explained that electrons can act like _____________________________. He also showed that electrons on circular orbits can only have _____________________ numbers of wavelengths. o de Broglie predicted that all moving particles have wave characteristics. de Broglie knew that if an electron act ...
... He explained that electrons can act like _____________________________. He also showed that electrons on circular orbits can only have _____________________ numbers of wavelengths. o de Broglie predicted that all moving particles have wave characteristics. de Broglie knew that if an electron act ...
DARLLENWCH Y DARN ISOD AC ATEBWCH Y CWESTIYNAU SY
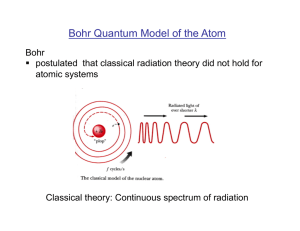
... for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mecha ...
... for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mecha ...
In 1913 Bohr proposed his quantized shell model of the atom to
... Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mechanics by itself could ...
... Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mechanics by itself could ...
Models of the Atom
... De Broglie Waves in Atoms Why should orbits be quantized a la Bohr? De Broglie; wave is associated with electron l = h/mv Only orbits that correspond to standing ...
... De Broglie Waves in Atoms Why should orbits be quantized a la Bohr? De Broglie; wave is associated with electron l = h/mv Only orbits that correspond to standing ...
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
(n=1).
... n = principal quantum number (1, 2, 3, …) l = angular momentum (0, 1, 2, … n-1) ml = component of l (-l < ml < l) ms = spin (-½ , +½) ...
... n = principal quantum number (1, 2, 3, …) l = angular momentum (0, 1, 2, … n-1) ml = component of l (-l < ml < l) ms = spin (-½ , +½) ...
CHAPTER 4 TEST REVIEW GUIDE
... 7. What is the difference between the Bohr model and the Quantum-Mechanical Model. ORBIT -> ORBITAL 7a. Explain the contributions of DeBroglie, Heisenberg and Schrodinger to our current understanding of the atom (quantum-mechanical model). ...
... 7. What is the difference between the Bohr model and the Quantum-Mechanical Model. ORBIT -> ORBITAL 7a. Explain the contributions of DeBroglie, Heisenberg and Schrodinger to our current understanding of the atom (quantum-mechanical model). ...
(Bohr Model And X-Rays) Part-1
... Bohr gave following postulates for electron in hydrogen atom :• An electron in an atom could resolve in certain stable orbits without the emission of radiant energy. • An electron resolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of L= ...
... Bohr gave following postulates for electron in hydrogen atom :• An electron in an atom could resolve in certain stable orbits without the emission of radiant energy. • An electron resolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of L= ...
Chapter 2: Data Analysis
... The light coming out of the excited atomic entities is very specific to ...
... The light coming out of the excited atomic entities is very specific to ...
Lesson 2 - The Bohr and Quantum Mechanical Model of the Atom
... The orbitals of electrons are determined statistically by creating a 3D electron probability density. Video: The Uncertain Location of Electrons ...
... The orbitals of electrons are determined statistically by creating a 3D electron probability density. Video: The Uncertain Location of Electrons ...
mp2b-16 honors
... What are the frequency, wavelength, and amplitude of a light wave? Be able to diagram. What happens when each is changed? ...
... What are the frequency, wavelength, and amplitude of a light wave? Be able to diagram. What happens when each is changed? ...
the Bohr`s atom model - Latin-American Journal of Physics Education
... electron in the orbit and the frequency of the radiation emitted in the “quantum jump” of the electron between orbits, which is known as the Principle of Correspondence. Using this principle, the first two postulates enunciated above, and Rydberg’s formula describing the energy of the observed (meas ...
... electron in the orbit and the frequency of the radiation emitted in the “quantum jump” of the electron between orbits, which is known as the Principle of Correspondence. Using this principle, the first two postulates enunciated above, and Rydberg’s formula describing the energy of the observed (meas ...
Problem Set 05
... A2. Spiral death time of an atom (in five easy steps): In Rutherford's planetary model of the atom electrons orbit around a very small massive nucleus. Classically, such an atom will have a finite lifetime due to radiative energy loss of the electrons, causing them to spiral in towards the nucleus. ...
... A2. Spiral death time of an atom (in five easy steps): In Rutherford's planetary model of the atom electrons orbit around a very small massive nucleus. Classically, such an atom will have a finite lifetime due to radiative energy loss of the electrons, causing them to spiral in towards the nucleus. ...
Homework Set 1
... any system is the most in need of a quantum description.) c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) classical Bohr orbit. d. Using the Bohr theory, calculate the ionization energi ...
... any system is the most in need of a quantum description.) c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) classical Bohr orbit. d. Using the Bohr theory, calculate the ionization energi ...
Bohr Model and Principal Quantum Number
... Once he saw Balmer’s work, Bohr developed his model Bohr postulated: 1. Electrons exist in circular orbits 2. Electrons exist only in allowed orbits 3. Electrons do not radiate energy within an orbit 4. Electrons can jump between orbits ...
... Once he saw Balmer’s work, Bohr developed his model Bohr postulated: 1. Electrons exist in circular orbits 2. Electrons exist only in allowed orbits 3. Electrons do not radiate energy within an orbit 4. Electrons can jump between orbits ...
Radiation and quantised orbits
... Question: Bohr rejected the Rutherford’s model of atom by arguing that it contradicts the classical theory of electrodynamics which says that a revolving charge should radiate energy. But in his model, Bohr directly assumed that the orbits in which electrons revolve are NON RADIATING. According to h ...
... Question: Bohr rejected the Rutherford’s model of atom by arguing that it contradicts the classical theory of electrodynamics which says that a revolving charge should radiate energy. But in his model, Bohr directly assumed that the orbits in which electrons revolve are NON RADIATING. According to h ...
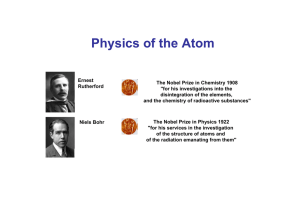
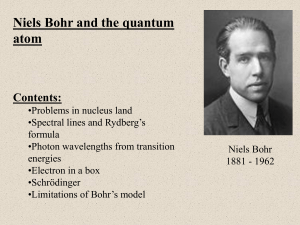
Niels Bohr

Niels Henrik David Bohr (Danish: [nels ˈb̥oɐ̯ˀ]; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Bohr was also a philosopher and a promoter of scientific research.Bohr developed the Bohr model of the atom, in which he proposed that energy levels of electrons are discrete and that the electrons revolve in stable orbits around the atomic nucleus but can jump from one energy level (or orbit) to another. Although the Bohr model has been supplanted by other models, its underlying principles remain valid. He conceived the principle of complementarity: that items could be separately analysed in terms of contradictory properties, like behaving as a wave or a stream of particles. The notion of complementarity dominated Bohr's thinking in both science and philosophy.Bohr founded the Institute of Theoretical Physics at the University of Copenhagen, now known as the Niels Bohr Institute, which opened in 1920. Bohr mentored and collaborated with physicists including Hans Kramers, Oskar Klein, George de Hevesy and Werner Heisenberg. He predicted the existence of a new zirconium-like element, which was named hafnium, after the Latin name for Copenhagen, where it was discovered. Later, the element bohrium was named after him.During the 1930s, Bohr helped refugees from Nazism. After Denmark was occupied by the Germans, he had a famous meeting with Heisenberg, who had become the head of the German nuclear energy project. In September 1943, word reached Bohr that he was about to be arrested by the Germans, and he fled to Sweden. From there, he was flown to Britain, where he joined the British Tube Alloys nuclear weapons project, and was part of the British mission to the Manhattan Project. After the war, Bohr called for international cooperation on nuclear energy. He was involved with the establishment of CERN and the Research Establishment Risø of the Danish Atomic Energy Commission, and became the first chairman of the Nordic Institute for Theoretical Physics in 1957.