* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Physics of the Atom
Particle in a box wikipedia , lookup
Double-slit experiment wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Elementary particle wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical bond wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
James Franck wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron scattering wikipedia , lookup
Electron configuration wikipedia , lookup
Tight binding wikipedia , lookup
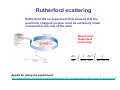
Physics of the Atom Ernest Rutherford Niels Bohr The Nobel Prize in Chemistry 1908 "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances" The Nobel Prize in Physics 1922 "for his services in the investigation of the structure of atoms and of the radiation emanating from them" Early Models of the Atom atoms : electrically neutral they can become charged positive and negative charges are around and some can be removed. popular atomic model “plum-pudding” model: Rutherford scattering Rutherford did an experiment that showed that the positively charged nucleus must be extremely small compared to the rest of the atom. Result from Rutherford scattering 2 ⎞2 dσ ⎛⎜ 1 Zze ⎟ 1 =⎜ dΩ ⎝ 4πε 0 4 K ⎟⎠ sin 4 (θ / 2 ) Applet for doing the experiment: http://www.physics.upenn.edu/courses/gladney/phys351/classes/Scattering/Rutherford_Scattering.htm l Rutherford scattering the smallness of the nucleus the radius of the nucleus is 1/10,000 that of the atom. the atom is mostly empty space Rutherford’s atomic model Atomic Spectra: Key to the Structure of the Atom A very thin gas heated in a discharge tube emits light only at characteristic frequencies. Atomic Spectra: Key to the Structure of the Atom Line spectra: absorption and emission The Balmer series in atomic hydrogen The wavelengths of electrons emitted from hydrogen have a regular pattern: Johann Jakob Balmer Lyman, Paschen and Rydberg series the Lyman series: the Paschen series: Janne Rydberg The Spectrum of the hydrogen Atom A portion of the complete spectrum of hydrogen is shown here. The lines cannot be explained by classical atomic theory. The Bohr Model Solution to radiative instability of the atom: - atom exists in a discrete set of stationary states no radiation when atom is in such state - radiative transitions Æ quantum jumps between levels hν = hc λ = Ei − E f - angular momentum is quantized . These are ad hoc hypotheses by Bohr, against intuitions of classical physics The Bohr Model: derivation An electron is held in orbit by the Coulomb force: (equals centripetal force) mv 2 Ze 2 = rn 4πε 0 rn2 Solve: the size of the orbit is quantized (and we know the size of an atom !) . Quantisation of energy 2 2 Ze Z 1 2 En = mv − = − 2 R∞ 2 4πε 0 rn n 2 ⎛ e 2 ⎞ me ⎟ R∞ = ⎜⎜ 2 ⎟ ⎝ 4πε 0 ⎠ 2h Rydberg constant EXTRA Reduced mass in the old Bohr model Æ the one particle problem Velocity vectors: r v1 = M r v m+M M r r v v2 = − m+M Relative coordinates: r r r r = r1 − r2 Centre of Mass Relative velocity r r dr v= dt Kinetic energy r r mr1 + Mr2 = 0 1 1 1 K = m1v12 + m2v22 = μv 2 2 2 2 Position vectors: Angular momentum r r1 = M r r m+M r r2 = − m r r m+M L = m1v1r1 + m2v2 r2 = μvr With reduced mass μ= mM m+M Centripetal force m1v12 m2v22 μv 2 F= = = r1 r2 r Quantisation of angular momentum: L = μvr = n h = nh 2π Results Quantisation of radius in orbit: n 2 4πε 0h 2 n 2 me rn = a0 = 2 Z e μ Z μ Energy levels in the Bohr model: Z2 ⎛ μ ⎞ En = − 2 ⎜⎜ ⎟⎟ R∞ n ⎝ me ⎠ Optical transitions in The Bohr Model The lowest energy level is called the ground state; the others are excited states. Calculate wavelengths and frequencies λ= c hc = f E2 − E1 Quantum states and kinetics Hydrogen at 20°C. Estimate the average kinetic energy of whole hydrogen atoms (not just the electrons) at room temperature, and use the result to explain why nearly all H atoms are in the ground state at room temperature, and hence emit no light. 3 K = k BT = 6.2 × 10 − 21 J = 0.04eV 2 Gas at elevated temperature – probability exited state is populated: Pn (T ) = e − En / kT Energy in gas: E = ∑ En e n ∑e n − En / kT − En / kT de Broglie’s Hypothesis Applied to Atoms Electron of mass m has a wave nature Electron in orbit: a standing wave 2πrn = nλ Substitution gives the quantum condition nh L = mvrn = 2π de Broglie’s Hypothesis Applied to Atoms These are circular standing waves for n = 2, 3, and 5. Standing waves do not radiate; Interpretation: electron does not move (no acceleration)