ch14
... SnCl2 and PbCl2 are white, crystalline solids with high melting points. SnCl4 is a volatile, benzene-soluble liquid. ...
... SnCl2 and PbCl2 are white, crystalline solids with high melting points. SnCl4 is a volatile, benzene-soluble liquid. ...
Chapters 18 – The Periodic Table
... The H – ion in LiH (and other column-I and -II hydrides) is a strong base, reacting with any molecule containing acidic H to form H2(g). These reactions can also be regarded as oxidationreduction. However, the reaction is much milder than most redox reactions, suggesting that it is best considered a ...
... The H – ion in LiH (and other column-I and -II hydrides) is a strong base, reacting with any molecule containing acidic H to form H2(g). These reactions can also be regarded as oxidationreduction. However, the reaction is much milder than most redox reactions, suggesting that it is best considered a ...
Chapter 1.1 –Chemistry is a Physical Science Chemistry is one of
... Vertical columns are called groups (18). Elements within a group have similar properties. ...
... Vertical columns are called groups (18). Elements within a group have similar properties. ...
Chapters 19 & 20
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
5.3 Representative Groups PPT
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
The subject of " Engineering Materials " deals with the study of
... state possess high thermal and electrical conductivity . the electrical resistance of pure metals increases with the temperature Many metals display duper conductivity at temperatures near absolute Zero where their electrical resistance drops abruptly to extremely low values . All metals are capable ...
... state possess high thermal and electrical conductivity . the electrical resistance of pure metals increases with the temperature Many metals display duper conductivity at temperatures near absolute Zero where their electrical resistance drops abruptly to extremely low values . All metals are capable ...
Chemistry: The Periodic Table and Periodicity
... What is the general trend of reactivity for non-metals within a group and a period? Why? Reactivity decreases as you go down a group, because there are more shells making it harder for the nucleus to pull electrons closer to the nucleus. Reactivity increases as you go across a period because electro ...
... What is the general trend of reactivity for non-metals within a group and a period? Why? Reactivity decreases as you go down a group, because there are more shells making it harder for the nucleus to pull electrons closer to the nucleus. Reactivity increases as you go across a period because electro ...
Chapter 5
... They’re very reactive They’re all silvery and soft enough to cut with a knife Not found in nature though because they’re so reactive Are usually stored in kerosene to keep them from reacting with the air or water ...
... They’re very reactive They’re all silvery and soft enough to cut with a knife Not found in nature though because they’re so reactive Are usually stored in kerosene to keep them from reacting with the air or water ...
C4C5C6
... points, low density Sodium + Water Sodium Hydroxide + Water and are very soft. Alkali metals burn with characteristic ...
... points, low density Sodium + Water Sodium Hydroxide + Water and are very soft. Alkali metals burn with characteristic ...
Section 2.5 Molecules and Ions
... Figure 2.15 is useful in learning oxoacids and their corresponding oxyanions. After students have been introduced to hypochlorous, chlorous, chloric, and perchloric acids, a challenging question is to ask them to describe components of HIO. Naming hydrates is challenging. Explain that we can use num ...
... Figure 2.15 is useful in learning oxoacids and their corresponding oxyanions. After students have been introduced to hypochlorous, chlorous, chloric, and perchloric acids, a challenging question is to ask them to describe components of HIO. Naming hydrates is challenging. Explain that we can use num ...
Periodic Table Vocabulary Alkali metals
... 21. Groups- Columns of elements help define element groups. Elements within a group share several common properties. Groups are elements have the same outer electron arrangement. Sentence- Group one is the alkali metals. ...
... 21. Groups- Columns of elements help define element groups. Elements within a group share several common properties. Groups are elements have the same outer electron arrangement. Sentence- Group one is the alkali metals. ...
Chapter 7 Periodic Properties of the Elements
... 17. Screening by the valence electrons in atoms is __________. (a). less efficient than that by core electrons (b). more efficient than that by core electrons (c). essentially identical to that by core electrons (d). both more efficient than that by core electrons and responsible for a general incre ...
... 17. Screening by the valence electrons in atoms is __________. (a). less efficient than that by core electrons (b). more efficient than that by core electrons (c). essentially identical to that by core electrons (d). both more efficient than that by core electrons and responsible for a general incre ...
File
... • B. A noble gas • C. A radioactive element • D. an alkalai metal • E. An alkaline earth metal ...
... • B. A noble gas • C. A radioactive element • D. an alkalai metal • E. An alkaline earth metal ...
The Periodic Table - Brookwood High School
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
Study Guide Answers
... elements in the same group have similar chemical and physical properties, What are families of the periodic table? There are 18 Families ( or groups), these are the vertical columns; elements in the same group have similar chemical and physical properties, What are at least 3 characteristics for: Al ...
... elements in the same group have similar chemical and physical properties, What are families of the periodic table? There are 18 Families ( or groups), these are the vertical columns; elements in the same group have similar chemical and physical properties, What are at least 3 characteristics for: Al ...
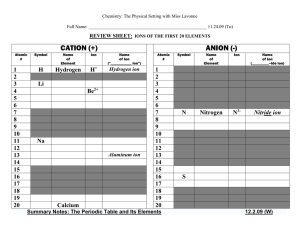
IONS OF THE FIRST 20 ELEMENTS
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
Unit 3 Revision Notes 213.00KB 2017-03-01 18
... 3) No lime scale is formed. There are 2 popular methods 1) Calcium ions may be taken out of solution by adding another ion to form an insoluble calcium salt. e.g. Sodium carbonate (washing soda) reacts to form insoluble calcium carbonate. 2) An ion exchange resin can be used. This is a material cont ...
... 3) No lime scale is formed. There are 2 popular methods 1) Calcium ions may be taken out of solution by adding another ion to form an insoluble calcium salt. e.g. Sodium carbonate (washing soda) reacts to form insoluble calcium carbonate. 2) An ion exchange resin can be used. This is a material cont ...
Rem001 - The Vital Chemist
... g. With water, calcium, strontium and barium react at 250c to form the respective hydroxides. Magnesium reacts at higher temperatures to form the oxide while beryllium does not react with water or steam. Ca + 2H2O ------→ Ca (OH)2 + H2 Mg + H2O-----→ MgO + H2 NOTE: Group IIa compounds are less solub ...
... g. With water, calcium, strontium and barium react at 250c to form the respective hydroxides. Magnesium reacts at higher temperatures to form the oxide while beryllium does not react with water or steam. Ca + 2H2O ------→ Ca (OH)2 + H2 Mg + H2O-----→ MgO + H2 NOTE: Group IIa compounds are less solub ...
Periodic Table of Elements
... _______________ metals are never found as free elements in nature. Elements that are ________________ bond easily with other elements to make ____________. All atoms, except hydrogen, want to have _______ electrons in their outermost energy level. This is called the rule of ...
... _______________ metals are never found as free elements in nature. Elements that are ________________ bond easily with other elements to make ____________. All atoms, except hydrogen, want to have _______ electrons in their outermost energy level. This is called the rule of ...
Study Guide Chapter 6
... Stability chart in the notebook). The Zones of Stability (see Zones of Stability chart in the notebook) shows that some electron configurations are more stable than others. The most stable electron configuration is a full outer level of electrons which is an octet for all levels except the first lev ...
... Stability chart in the notebook). The Zones of Stability (see Zones of Stability chart in the notebook) shows that some electron configurations are more stable than others. The most stable electron configuration is a full outer level of electrons which is an octet for all levels except the first lev ...
The Periodic Table
... He studied the patterns of the elements. He was able to organize the elements at the time in order of their atomic mass. He realized that the physical and chemical properties of elements were related to their atomic mass in a ‘periodic’ way. He arranged them so that groups of elements with similar p ...
... He studied the patterns of the elements. He was able to organize the elements at the time in order of their atomic mass. He realized that the physical and chemical properties of elements were related to their atomic mass in a ‘periodic’ way. He arranged them so that groups of elements with similar p ...
AP Chemistry Summer Assignment
... element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gase ...
... element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gase ...
The Periodic Table - Ms. Simmons
... Main Group Elements • most clearly illustrate periodic patterns • most abundant naturally occurring elements • most important elements for living things, including most common components of organisms: O, C, N, H, P, Ca ...
... Main Group Elements • most clearly illustrate periodic patterns • most abundant naturally occurring elements • most important elements for living things, including most common components of organisms: O, C, N, H, P, Ca ...