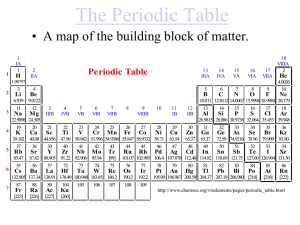
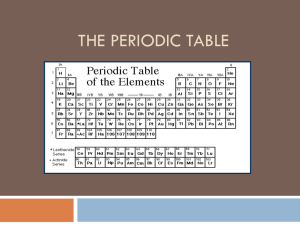
The Periodic Law Notes (Chapter 5) – Part 2
... your book) instead of the predicted configuration for these elements. You do NOT have to memorize these, they will be highlighted or marked on your periodic table. ...
... your book) instead of the predicted configuration for these elements. You do NOT have to memorize these, they will be highlighted or marked on your periodic table. ...
Periodic_Tendancies
... Down the Periodic Table •Family: Are arranged vertically down the periodic table (columns or group, 1- 18 or 1-8 A,B) •These elements have the same number electrons in the outer most shells, the valence shell. ...
... Down the Periodic Table •Family: Are arranged vertically down the periodic table (columns or group, 1- 18 or 1-8 A,B) •These elements have the same number electrons in the outer most shells, the valence shell. ...
questions on periodic classification of elements
... of the bond formed between them? To which period of the Modern periodic table do these two elements belong? 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has ...
... of the bond formed between them? To which period of the Modern periodic table do these two elements belong? 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has ...
The Periodic Table assignment
... date assigned_____________ date due ______________ date returned _____________ ...
... date assigned_____________ date due ______________ date returned _____________ ...
Year 9 study the new AQA GCSE specification for first examination
... In Group 7, the reactivity of the elements decreases going down the group. A more reactive halogen can displace a less reactive halogen from an aqueous solution of its salt. Students should be able to: • explain how properties of the elements in Group 7 depend on the outer shell of electrons o ...
... In Group 7, the reactivity of the elements decreases going down the group. A more reactive halogen can displace a less reactive halogen from an aqueous solution of its salt. Students should be able to: • explain how properties of the elements in Group 7 depend on the outer shell of electrons o ...
Periodic Table Notes 1
... 1. Name three categories that are used to classify elements in the periodic table. ...
... 1. Name three categories that are used to classify elements in the periodic table. ...
Periodic Table Assessment Quiz 2016
... element will have the atomic number, atomic weight, name, and symbol for each element. ...
... element will have the atomic number, atomic weight, name, and symbol for each element. ...
Ch 4-1 Notes
... Properties of hydrogen do not resemble those of any other group. Helium has two electrons in the s sublevel of the first energy level. It is part of group 18 (noble gases). Its filled s sublevel gives it special stability like the other members of group 18. Different than group 2 metals since they h ...
... Properties of hydrogen do not resemble those of any other group. Helium has two electrons in the s sublevel of the first energy level. It is part of group 18 (noble gases). Its filled s sublevel gives it special stability like the other members of group 18. Different than group 2 metals since they h ...
Periodic Law
... Periodic Law – when elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery c ...
... Periodic Law – when elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery c ...
Periodic Classification of Elements
... always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner. It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an ...
... always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner. It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an ...
Chapter 5 - EZWebSite
... electrons to the same energy level, but you also add more protons to the nucleus. More protons exerts a stronger attractive force and pulls the electrons in more tightly. What happens to atomic radius as you go down a group? ...
... electrons to the same energy level, but you also add more protons to the nucleus. More protons exerts a stronger attractive force and pulls the electrons in more tightly. What happens to atomic radius as you go down a group? ...
Periodic Table Notes 2
... in a meaningful way. He developed classification scheme based upon molar masses. Elements that showed similar patterns of chemical behavior were placed into groups. His chart was incomplete at the time, but Mendeleev predicted that new elements were yet to be discovered, so he left blank spaces in h ...
... in a meaningful way. He developed classification scheme based upon molar masses. Elements that showed similar patterns of chemical behavior were placed into groups. His chart was incomplete at the time, but Mendeleev predicted that new elements were yet to be discovered, so he left blank spaces in h ...
The Periodic Table of Elements
... want to get rid of it to be stable (octet in energy level below) Alkaline earth metals are less reactive than alkali metals Alkaline earth metals are not found pure in nature; they are too reactive Found in Earth’s crust in mineral form. ...
... want to get rid of it to be stable (octet in energy level below) Alkaline earth metals are less reactive than alkali metals Alkaline earth metals are not found pure in nature; they are too reactive Found in Earth’s crust in mineral form. ...
PERIODIC TABLE
... In their pure state, all of the alkali metals have a silvery appearance and are soft enough to cut with a knife. ...
... In their pure state, all of the alkali metals have a silvery appearance and are soft enough to cut with a knife. ...
03 Chapter 2 Atomic Structure Power point Periodic Table
... The energy required to remove the first electron from a gaseous atom. Second ionization removes the second electron and so on. Can be used to predict ionic charges. ...
... The energy required to remove the first electron from a gaseous atom. Second ionization removes the second electron and so on. Can be used to predict ionic charges. ...
MATTER AND PERIODIC TABLE
... positively charged protons and neutral neutrons. A cloud of negatively charged electrons surrounds the nucleus of an atom. ...
... positively charged protons and neutral neutrons. A cloud of negatively charged electrons surrounds the nucleus of an atom. ...
The Periodic Table
... Periodic law states that when elements are ordered by increasing atomic number, their chemical and physical properties repeat in a pattern. ...
... Periodic law states that when elements are ordered by increasing atomic number, their chemical and physical properties repeat in a pattern. ...
How the Periodic Table Works
... chemical reactions. Although they're less reactive than alkali metals, they're not usually found alone in nature. For example, calcium combines with carbon to make calcium carbonate, which makes up limestone, marble and seashells. Teeth and bones are also made of calcium compounds. Beryllium contrib ...
... chemical reactions. Although they're less reactive than alkali metals, they're not usually found alone in nature. For example, calcium combines with carbon to make calcium carbonate, which makes up limestone, marble and seashells. Teeth and bones are also made of calcium compounds. Beryllium contrib ...
alkali metal
... Dmitri Mendeleev in 1869. The amazing accuracy of his predictions has been very important to chemists in this century. However, the basis of his arrangement was the atomic masses of the elements. This approach proved incorrect as it would have placed some elements in a group with dissimilar properti ...
... Dmitri Mendeleev in 1869. The amazing accuracy of his predictions has been very important to chemists in this century. However, the basis of his arrangement was the atomic masses of the elements. This approach proved incorrect as it would have placed some elements in a group with dissimilar properti ...
Science Questions
... Reason: Any metal will combine chemically with any non-metal to form ionic bonds that hold the molecule together. ...
... Reason: Any metal will combine chemically with any non-metal to form ionic bonds that hold the molecule together. ...
Chapter 5 – The Periodic Law
... Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements. Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic number. Describe the relationship between electrons in orb ...
... Describe the modern periodic table Explain how the periodic law can be used to predict the physical and chemical properties of elements. Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic number. Describe the relationship between electrons in orb ...
Practice Questions
... (1) filled principal energy levels (2) occupied principal energy levels (3) neutrons in the nucleus (4) electrons in the valence shell 20. Which of the Group 15 elements can lose an ...
... (1) filled principal energy levels (2) occupied principal energy levels (3) neutrons in the nucleus (4) electrons in the valence shell 20. Which of the Group 15 elements can lose an ...
Document
... Chemical reaction- process where one or more substances are rearranged to form new substances ...
... Chemical reaction- process where one or more substances are rearranged to form new substances ...
Physical Science
... Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy l ...
... Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy l ...