Periodic Table web
... Water, Fire, and Earth Scientists have identified 90 naturally occurring elements, and created about 28 others ...
... Water, Fire, and Earth Scientists have identified 90 naturally occurring elements, and created about 28 others ...
REACTION PREDICTION
... Double Replacement (metathesis) Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
... Double Replacement (metathesis) Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
Regents Chemistry NOTE PACKET
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
S8P1-study-guide
... particles that carry a positive charge. Neutrons are particles that carry no electric charge. The nucleus is the center of the atom that is made up of both protons and neutrons. Electrons are particles that carry a negative charge and surround the nucleus in what is know as the electron cloud on ene ...
... particles that carry a positive charge. Neutrons are particles that carry no electric charge. The nucleus is the center of the atom that is made up of both protons and neutrons. Electrons are particles that carry a negative charge and surround the nucleus in what is know as the electron cloud on ene ...
THE PERIODIC TABLE Introduction • Dmitri Mendeleev is the father
... • In the periodic table, the metalloids lie along the border between metals and nonmetals. • Some metalloids such as silicon, germanium (Ge), and arsenic (As) are semiconductors. • Metalloids have some chemical and physical properties of metals and other properties of nonmetals. 3. Atomic Radius • A ...
... • In the periodic table, the metalloids lie along the border between metals and nonmetals. • Some metalloids such as silicon, germanium (Ge), and arsenic (As) are semiconductors. • Metalloids have some chemical and physical properties of metals and other properties of nonmetals. 3. Atomic Radius • A ...
The Periodic Law Notes (Chapter 5) – Part 2
... your book) instead of the predicted configuration for these elements. You do NOT have to memorize these, they will be highlighted or marked on your periodic table. ...
... your book) instead of the predicted configuration for these elements. You do NOT have to memorize these, they will be highlighted or marked on your periodic table. ...
periodictrendsss - rlsciencecurriculum
... or her notebook paper explaining how the cards were arranged and why you decided to use that particular arrangement. PRINT ONE COPY FOR EACH GROUP MEMBER. BE SURE TO PUT THE NAMES OF ALL GROUP MEMBERS ON THE DOCUMENT. ...
... or her notebook paper explaining how the cards were arranged and why you decided to use that particular arrangement. PRINT ONE COPY FOR EACH GROUP MEMBER. BE SURE TO PUT THE NAMES OF ALL GROUP MEMBERS ON THE DOCUMENT. ...
Study Guide
... particles that carry a positive charge. Neutrons are particles that carry no electric charge. The nucleus is the center of the atom that is made up of both protons and neutrons. Electrons are particles that carry a negative charge and surround the nucleus in what is know as the electron cloud on ene ...
... particles that carry a positive charge. Neutrons are particles that carry no electric charge. The nucleus is the center of the atom that is made up of both protons and neutrons. Electrons are particles that carry a negative charge and surround the nucleus in what is know as the electron cloud on ene ...
The Periodic Table of the Elements
... • Most elements do not exist in their ‘pure’ form but combine with other elements to form substances with properties different from the elements themselves. • A compound is a substance that consists of two or more elements that are chemically combined in specific proportions. • Compounds are formed ...
... • Most elements do not exist in their ‘pure’ form but combine with other elements to form substances with properties different from the elements themselves. • A compound is a substance that consists of two or more elements that are chemically combined in specific proportions. • Compounds are formed ...
reviewing key trends
... Complete the table on the back side of this sheet, labeled “Families of Elements.” Where are the lanthanoids located, and how are they similar? Where are the actinoids, and how are they similar? What is a similarity between alkali metals and alkaline earth metals? ...
... Complete the table on the back side of this sheet, labeled “Families of Elements.” Where are the lanthanoids located, and how are they similar? Where are the actinoids, and how are they similar? What is a similarity between alkali metals and alkaline earth metals? ...
Group 1: The Alkali Metals
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
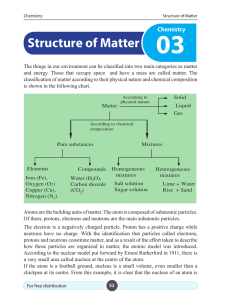
Structure of Matter - e
... The atomic number is the number of protons in an atom of the element. Atomic number of the element = number of protons in an atom of the element For example, there are 11 protons in the nucleus of a sodium atom. Thus, the atomic number of sodium is 11.The number of protons in every atom of the same ...
... The atomic number is the number of protons in an atom of the element. Atomic number of the element = number of protons in an atom of the element For example, there are 11 protons in the nucleus of a sodium atom. Thus, the atomic number of sodium is 11.The number of protons in every atom of the same ...
Chemistry Spell check on
... 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one co ...
... 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one co ...
Word - Chemistry and More
... 1. (Chapter 2) Identify the following properties as physical or chemical properties: a) Copper is shiny and orange. b) Potassium reacts explosively with fluorine gas to produce potassium fluoride. c) Oxygen is a gas at room temperature. d) Sodium oxide has a very high melting point. e) Sodium chlori ...
... 1. (Chapter 2) Identify the following properties as physical or chemical properties: a) Copper is shiny and orange. b) Potassium reacts explosively with fluorine gas to produce potassium fluoride. c) Oxygen is a gas at room temperature. d) Sodium oxide has a very high melting point. e) Sodium chlori ...
Ex. 41 Answer
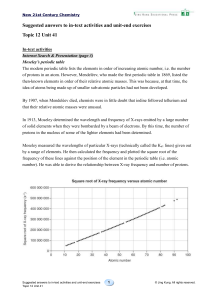
... is greater. The electrons are pulled closer to the nucleus. So, the atomic radius of F is smaller than that of O. O and S belong to the same group. An atom of S has 1 more occupied electron shell than an atom of O and the shielding effect of the inner shell electrons is greater. Thus, compared with ...
... is greater. The electrons are pulled closer to the nucleus. So, the atomic radius of F is smaller than that of O. O and S belong to the same group. An atom of S has 1 more occupied electron shell than an atom of O and the shielding effect of the inner shell electrons is greater. Thus, compared with ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
Unit 3.2 Periodic Table Test
... An electron has the same mass as a proton. An electron has much more mass than a neutron. An electron has about the same mass as a neutron. An electron has much less mass than a proton. An electron has much less mass than a neutron. A proton has more mass than an electron. A proton has less mass tha ...
... An electron has the same mass as a proton. An electron has much more mass than a neutron. An electron has about the same mass as a neutron. An electron has much less mass than a proton. An electron has much less mass than a neutron. A proton has more mass than an electron. A proton has less mass tha ...
AQA Additional Sci C2 Revision Guide
... When melted or dissolved in water, ionic compounds conduct electricity because the ions are free to move and carry the current. This provides evidence that these compounds are actually made up of ions. ...
... When melted or dissolved in water, ionic compounds conduct electricity because the ions are free to move and carry the current. This provides evidence that these compounds are actually made up of ions. ...
Chapter 5 Reading Guide Please answer the following questions in
... At quick glance of the Mendeleev’s periodic table (bottom of page 127), what are some differences you spot from the modern periodic table (like the one on the wall or in your ...
... At quick glance of the Mendeleev’s periodic table (bottom of page 127), what are some differences you spot from the modern periodic table (like the one on the wall or in your ...
PROFESSIONAL LEARNING COMMUNITY MODEL FOR ENTRY
... Prominent groups include alkali metals, alkaline earth metals, halogens, and noble gases. Regions of the periodic table are also broken up into two different regions: main group (or representative) elements and transition elements. An 'unknown' element's properties can be identified by examining the ...
... Prominent groups include alkali metals, alkaline earth metals, halogens, and noble gases. Regions of the periodic table are also broken up into two different regions: main group (or representative) elements and transition elements. An 'unknown' element's properties can be identified by examining the ...
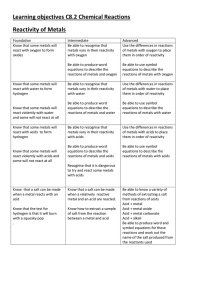
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... Acid + metal Acid + metal oxide Acid + metal carbonate Acid + alkali Be able to produce word and symbol equations for these reactions and work out the name of the salt produced from the reactants used ...
... Acid + metal Acid + metal oxide Acid + metal carbonate Acid + alkali Be able to produce word and symbol equations for these reactions and work out the name of the salt produced from the reactants used ...
TREND
... 15. What periodic trends exist for ionization energy? How about for electron affinity? What about atomic radius and its trend? 16. Why does the first period of the periodic table contain only two elements while all the other periods have eight or more element in them? 17 What feature of electron con ...
... 15. What periodic trends exist for ionization energy? How about for electron affinity? What about atomic radius and its trend? 16. Why does the first period of the periodic table contain only two elements while all the other periods have eight or more element in them? 17 What feature of electron con ...
The Periodic Table
... Number of valence electrons each atom has When outer levels are full, atoms are stable When they are not full, they react: gain, lose, or share 1 or 2 electrons ...
... Number of valence electrons each atom has When outer levels are full, atoms are stable When they are not full, they react: gain, lose, or share 1 or 2 electrons ...
CH 5 Section Review 1-3
... 2. The length of each period in the periodic table is determined by the (a) atomic masses of the elements; (b) atomic numbers of the elements; (c) sublevels being filled with electrons; (d) number of isotopes of each element. ...
... 2. The length of each period in the periodic table is determined by the (a) atomic masses of the elements; (b) atomic numbers of the elements; (c) sublevels being filled with electrons; (d) number of isotopes of each element. ...
Station 1: The Periodic Table 1a. Students know how to relate the
... How are groups identified? o Groups can be identified in two different ways. By number and name. Group 1 is also known as the Alkali Metals (except H). Group 2 is known as the Alkaline Earth Metals. Group 17 is known as the Halogens. Group 18 is known as the Noble Gases. o Elements that ...
... How are groups identified? o Groups can be identified in two different ways. By number and name. Group 1 is also known as the Alkali Metals (except H). Group 2 is known as the Alkaline Earth Metals. Group 17 is known as the Halogens. Group 18 is known as the Noble Gases. o Elements that ...