The Quantum Mechanical Model of the Atom
... • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a particular value of E. – A specific wave function is often called an orbital. ...
... • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a particular value of E. – A specific wave function is often called an orbital. ...
Syllabus
... The main objective of this course is to examine the theoretical basis for our present understanding of the structure of matter at the atomic and molecular level. To that end we will review those aspects of quantum mechanics that play the most important role in this understanding. This includes the s ...
... The main objective of this course is to examine the theoretical basis for our present understanding of the structure of matter at the atomic and molecular level. To that end we will review those aspects of quantum mechanics that play the most important role in this understanding. This includes the s ...
Document
... from Niels Bohr who explained experimentally observed discrete nature of atomic spectrum of Hydrogen. In spite of its immediate success in providing theoretical account of the spectrum and other nature of Hydrogen atom, a complete understanding of Bohr’s atom came only after de Broglie’s conjecture ...
... from Niels Bohr who explained experimentally observed discrete nature of atomic spectrum of Hydrogen. In spite of its immediate success in providing theoretical account of the spectrum and other nature of Hydrogen atom, a complete understanding of Bohr’s atom came only after de Broglie’s conjecture ...
HOMEWORK 4-4 - losbanosusd.org
... STANDARDIZED TEST PREP Circle the letter of the best answer. 1. Which of the following indicates the s sublevel in the third main energy level? a. s3 b. 3xyz ...
... STANDARDIZED TEST PREP Circle the letter of the best answer. 1. Which of the following indicates the s sublevel in the third main energy level? a. s3 b. 3xyz ...
The quantum mechanics of photon addition and subtraction
... composed of photons, optical packets so small that a typical laser pointer with 1mW of power emits billions of them each second. Being able to take away or add a single photon in a light field at will would be useful for accurate engineering of quantum states. Such pure single photons are ideal for ...
... composed of photons, optical packets so small that a typical laser pointer with 1mW of power emits billions of them each second. Being able to take away or add a single photon in a light field at will would be useful for accurate engineering of quantum states. Such pure single photons are ideal for ...
Electron Configuration and Chemical Periodicity
... equation are available – Electron-electron interactions are important – The same tree quantum numbers (n, l and ml) are used to describe the solutions (the orbitals are hydrogen-like) ...
... equation are available – Electron-electron interactions are important – The same tree quantum numbers (n, l and ml) are used to describe the solutions (the orbitals are hydrogen-like) ...
Isra University Faculty of Arts and science Course Calendar 2016
... of the case for the transition of dual-electrode, properties, transit behavior for the transition of dualelectrode, the laser and its applications, reverse rehabilitation, Springs Q, laser four levels. * Objectives: ...
... of the case for the transition of dual-electrode, properties, transit behavior for the transition of dualelectrode, the laser and its applications, reverse rehabilitation, Springs Q, laser four levels. * Objectives: ...
Quantum Mechanics - s3.amazonaws.com
... electron, and a thin wall or barrier, the electron may actually tunnel through the barrier. The solution to the bound particle in a finite well had the wavefunction decaying exponentially in the wall. If the wall is thin, there is a non-zero amplitude to the wavefunction at x=L. ...
... electron, and a thin wall or barrier, the electron may actually tunnel through the barrier. The solution to the bound particle in a finite well had the wavefunction decaying exponentially in the wall. If the wall is thin, there is a non-zero amplitude to the wavefunction at x=L. ...
Quantum Information (QI) - BYU Physics and Astronomy
... What will we do? CP, HW, IP, MT, F How do you communicate? Sources for QI? (Linear Algebra prerequisite) What is Linear Algebra? ...
... What will we do? CP, HW, IP, MT, F How do you communicate? Sources for QI? (Linear Algebra prerequisite) What is Linear Algebra? ...
Unit 2: Atoms and their Electrons
... energy level first and then up, Pauli Exclusion principle states that no two electrons in an atom can have all four quantum numbers the same, Hund’s rule states that electrons do not pair up to fill an orbital in a sublevel until all orbitals in the same sublevel are half filled. 1s22s22p63s23p64s2 ...
... energy level first and then up, Pauli Exclusion principle states that no two electrons in an atom can have all four quantum numbers the same, Hund’s rule states that electrons do not pair up to fill an orbital in a sublevel until all orbitals in the same sublevel are half filled. 1s22s22p63s23p64s2 ...
Chapter 7 Quantum Theory of the Atom
... Building on de Broglie’s work, in 1926, Erwin Schrödinger devised a theory that could be used to explain the wave properties of electrons in atoms and molecules. The branch of physics that mathematically describes the wave properties of submicroscopic particles is called quantum mechanics or wave m ...
... Building on de Broglie’s work, in 1926, Erwin Schrödinger devised a theory that could be used to explain the wave properties of electrons in atoms and molecules. The branch of physics that mathematically describes the wave properties of submicroscopic particles is called quantum mechanics or wave m ...
CHEM 532 Physical Chemistry II (Quantum Chemistry) Fall 2013
... Students found responsible for academic integrity violations may receive an F on the particular assignment or exam, as well as an F for the course. Repeated and/or serious offenses may result in referral to the conduct board and expulsion from WSU. For graduate students, academic integrity violation ...
... Students found responsible for academic integrity violations may receive an F on the particular assignment or exam, as well as an F for the course. Repeated and/or serious offenses may result in referral to the conduct board and expulsion from WSU. For graduate students, academic integrity violation ...
Electron Orbits
... theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin ...
... theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin ...
Energy levels, photons and spectral lines
... Niels Bohr developed a model of the atom where the electrons had certain stable states that had quantized radii and energy ...
... Niels Bohr developed a model of the atom where the electrons had certain stable states that had quantized radii and energy ...
The 17st June 2009 This file is intended to provide more information
... this is known within 1 or 2% we don’t need to care too much, since the energy scans will be fast enough to cover that kind of energy domain. If the absolute value is not known to better than 5%, we’ll have to foresee some measurement. It can be done by the floating wire method. I just write here a h ...
... this is known within 1 or 2% we don’t need to care too much, since the energy scans will be fast enough to cover that kind of energy domain. If the absolute value is not known to better than 5%, we’ll have to foresee some measurement. It can be done by the floating wire method. I just write here a h ...
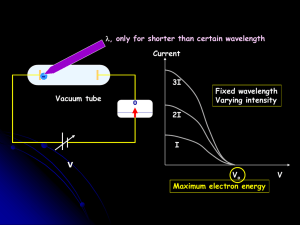
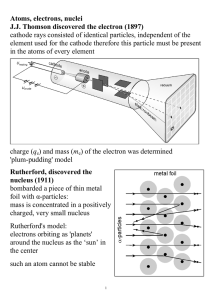
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a gen ...
... where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a gen ...
A Measurement of the Energy of Internal Conversion Electrons from
... examine the radial probability density distributions of the atomic electrons as predicted for a hydrogen atom from the solution to the Schrödinger equation. It is noted that there is a non-zero probability that the electron can be found within the physical boundary of the nucleus. The likelihood tha ...
... examine the radial probability density distributions of the atomic electrons as predicted for a hydrogen atom from the solution to the Schrödinger equation. It is noted that there is a non-zero probability that the electron can be found within the physical boundary of the nucleus. The likelihood tha ...
lecture31
... • “Allowed” transitions between energy levels occur between states whose value of l differ by one: • Other, “forbidden,” transitions also occur but with much lower probability • Photon has a spin angular momentum of 1ħ ...
... • “Allowed” transitions between energy levels occur between states whose value of l differ by one: • Other, “forbidden,” transitions also occur but with much lower probability • Photon has a spin angular momentum of 1ħ ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.