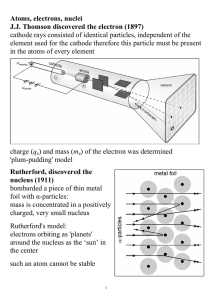
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
PROBABILITY THEORY 1. Prove that, if A and B are two events
... a pair of statistically independent events. Prove that, if A and B are two events, then the probability of the occurrence of either or both of them is given by P (A ∪ B) = P (A) + P (B) − P (A ∩ B). A population of fruit flies contains four phenotypes AB, Ab, aB, ab characterised by wing shape (A or ...
... a pair of statistically independent events. Prove that, if A and B are two events, then the probability of the occurrence of either or both of them is given by P (A ∪ B) = P (A) + P (B) − P (A ∩ B). A population of fruit flies contains four phenotypes AB, Ab, aB, ab characterised by wing shape (A or ...
Quantum Mechanics
... • It didn’t do a very good job of explaining how ions formed. • Bohr was able to improve on his 1913 model, but he needed Wolfgang Pauli to really make sense of it. ...
... • It didn’t do a very good job of explaining how ions formed. • Bohr was able to improve on his 1913 model, but he needed Wolfgang Pauli to really make sense of it. ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... by the columbic force of attraction between the proton and the electron. e2 - If we equate coulomb’s force law 2 with Eq.(1-21) 4 r ...
... by the columbic force of attraction between the proton and the electron. e2 - If we equate coulomb’s force law 2 with Eq.(1-21) 4 r ...
De Broglie Waves, Uncertainty, and Atoms
... • Electrons produce interference pattern just like light waves. – Need electrons to go through both slits. – What if we send 1 electron at a time? – Does a single electron go through both slits? ...
... • Electrons produce interference pattern just like light waves. – Need electrons to go through both slits. – What if we send 1 electron at a time? – Does a single electron go through both slits? ...
Chapter 4
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
No Slide Title - Rubin Gulaboski
... Electron Spin and the Pauli Exclusion Principle • Line spectra of many electron atoms show each line as a closely spaced pair of lines. • Stern and Gerlach designed an experiment to determine why. • A beam of atoms was passed through a slit and into a magnetic field and the atoms were then detected ...
... Electron Spin and the Pauli Exclusion Principle • Line spectra of many electron atoms show each line as a closely spaced pair of lines. • Stern and Gerlach designed an experiment to determine why. • A beam of atoms was passed through a slit and into a magnetic field and the atoms were then detected ...
CYK/2006/PH406/Tutorial 5 1. Calculate the probability of excitation
... 8. Generalise Einstein’s results in case the two levels E a and Eb are degenerate with degeneracies g a and gb respectively. 9. State and prove the Thomas-Reiche-Kuhn sum rule for oscillator strengths. 10. Calculate the Einstein’s coefficient A for the 2p - 1s transition in a hydrogenic atom, and fi ...
... 8. Generalise Einstein’s results in case the two levels E a and Eb are degenerate with degeneracies g a and gb respectively. 9. State and prove the Thomas-Reiche-Kuhn sum rule for oscillator strengths. 10. Calculate the Einstein’s coefficient A for the 2p - 1s transition in a hydrogenic atom, and fi ...
1 Niels Bohr`s semi-classical model (1913) 2 QM atomic shell model
... body theory shows that the electron density of an atom is the sum of the probability densities for all occupied quantum states. This suggests that the total density of an N-electron atom (which can be measured) might reveal the shell structure of the occupied orbitals! This is indeed the case. We wi ...
... body theory shows that the electron density of an atom is the sum of the probability densities for all occupied quantum states. This suggests that the total density of an N-electron atom (which can be measured) might reveal the shell structure of the occupied orbitals! This is indeed the case. We wi ...
Modern Physics - Politechnika Wrocławska
... radiation to suggest that in fact the quanta of energy used in blackbody radiation are in fact localised “particle like” energy packets Each having an energy given by hf Emitted electrons will have an energy given by ...
... radiation to suggest that in fact the quanta of energy used in blackbody radiation are in fact localised “particle like” energy packets Each having an energy given by hf Emitted electrons will have an energy given by ...
Electron Configuration Class Notes
... - This is due to the angular momentum of the electronic orbitals. - There are actually more than just the four quantum numbers - these other quantum numbers account for the extra variations. ***Be able to give the inert gas core configuration, the set of four quantum numbers for any electron, and th ...
... - This is due to the angular momentum of the electronic orbitals. - There are actually more than just the four quantum numbers - these other quantum numbers account for the extra variations. ***Be able to give the inert gas core configuration, the set of four quantum numbers for any electron, and th ...
PPT
... Photons (or electrons …) can produce interference patterns even one at a time ! With one slit closed, the image formed is simply a single-slit pattern. We “know” (i.e., we have constrained) which way the particle went. With both slits open, a particle interferes with itself to produce the observed t ...
... Photons (or electrons …) can produce interference patterns even one at a time ! With one slit closed, the image formed is simply a single-slit pattern. We “know” (i.e., we have constrained) which way the particle went. With both slits open, a particle interferes with itself to produce the observed t ...
Lecture 5
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
The Future of Computer Science
... Factoring is in BQP, but not believed to be NP-complete! Today, we don’t believe quantum computers can solve NP-complete problems in polynomial time in general (though not surprisingly, we can’t prove it) Bennett et al. 1997: “Quantum magic” won’t be enough If you throw away the problem structure, ...
... Factoring is in BQP, but not believed to be NP-complete! Today, we don’t believe quantum computers can solve NP-complete problems in polynomial time in general (though not surprisingly, we can’t prove it) Bennett et al. 1997: “Quantum magic” won’t be enough If you throw away the problem structure, ...
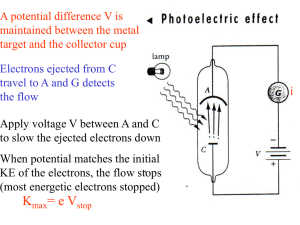
V stop f
... • Vstop does not depend on the intensity of the light source for a given frequency f • => classical physics would predict that if we increase the amplitude of the alternating electric field, then a larger kick would be given to the electron? • => if light is composed of photons, then the maximum ene ...
... • Vstop does not depend on the intensity of the light source for a given frequency f • => classical physics would predict that if we increase the amplitude of the alternating electric field, then a larger kick would be given to the electron? • => if light is composed of photons, then the maximum ene ...
m L
... Why Does the Bohr Model Fail? • Bohr’s model conflicts with the uncertainty principle because if the electron is set within a confined orbit, you know both its momentum and position at a given moment. Therefore, it violates the Uncertainty Principle and can not hold true. ...
... Why Does the Bohr Model Fail? • Bohr’s model conflicts with the uncertainty principle because if the electron is set within a confined orbit, you know both its momentum and position at a given moment. Therefore, it violates the Uncertainty Principle and can not hold true. ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.