* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy levels, photons and spectral lines
X-ray photoelectron spectroscopy wikipedia , lookup
James Franck wikipedia , lookup
Bell's theorem wikipedia , lookup
Quantum field theory wikipedia , lookup
Coherent states wikipedia , lookup
Tight binding wikipedia , lookup
Casimir effect wikipedia , lookup
Many-worlds interpretation wikipedia , lookup
Quantum machine learning wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Quantum group wikipedia , lookup
Quantum teleportation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
EPR paradox wikipedia , lookup
Particle in a box wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Atomic orbital wikipedia , lookup
Quantum state wikipedia , lookup
Double-slit experiment wikipedia , lookup
Renormalization wikipedia , lookup
Matter wave wikipedia , lookup
Renormalization group wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Electron configuration wikipedia , lookup
Canonical quantization wikipedia , lookup
History of quantum field theory wikipedia , lookup
Quantum key distribution wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Hidden variable theory wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
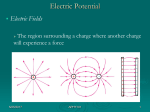
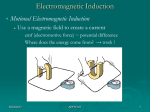
Quantum Physics Quantum The smallest quantity of a substance that still has the specific properties of that substance • Discrete vs. Continuous Albert Einstein showed that light is quantized and contains photons • Packets of energy 5/24/2017 APHY101 1 Quantum Physics Balmer Series How did physicists determine that the energy levels of electrons are quantized? • • • • Isaac Newton – prism and sunlight → light is a wave Interference patterns like with water → light is a wave Joseph von Fraunhofer – the Sun’s spectrum has gaps → ? Observations of gas emission and absorption spectrum → ? J. J. Balmer develops an equation that analyzes the pattern on the spectral lines that are observed. 5/24/2017 APHY101 2 Quantum Physics 5/24/2017 APHY101 3 Quantum Physics Photons Max Planck showed how the radiation emitted or absorbed by an object was quantized but still thought of light as a wave. • Einstein showed that photons have energy E = hf • Confirmed though experimentation by Robert Millikan Since h = 6.63 x 10-34 Js, each photon carries a very small amount of energy 5/24/2017 APHY101 4 Quantum Physics Photoelectric Effect Shining light on metal could cause electrons to be ejected from the metal. • This could not be explained using the wave theory of light Einstein’s photon theory of light explained the observations concerning the ejection of electrons from metals. • Waves vs. Photons 5/24/2017 APHY101 5 Quantum Physics The Bohr atom Thomson, Rutherford and Millikan discover the structure of the atom Classical EM theory could not explain this structure Niels Bohr developed a model of the atom where the electrons had certain stable states that had quantized radii and energy • Bound states of the electron in hydrogen 5/24/2017 APHY101 6 Quantum Physics Energy levels, photons and spectral lines Bohr’s model of the atom matches the observations of spectral lines from hydrogen • “Jumping” of the electron between energy levels Einstein and Planck explained how a photon is emitted or absorbed by an atom 5/24/2017 APHY101 7 Quantum Physics Energy levels, photons and spectral lines 5/24/2017 APHY101 8 Quantum Physics Energy levels, photons and spectral lines 5/24/2017 APHY101 9 Quantum Physics Energy levels, photons and spectral lines Louis de Broglie showed that electrons are standing waves that have mass and charge • They overlap the Bohr “orbits” • Explains why electron energy levels are quantized and why electrons do not spiral into the positive nucleus 5/24/2017 APHY101 10 Quantum Physics Energy levels, photons and spectral lines 5/24/2017 APHY101 11 Quantum Physics Elements Many substances but few “building blocks” • Think of the number of words and letters in English The Periodic Table • Tells us about the structure of elements and how they behave 5/24/2017 APHY101 12 Quantum Physics Elements Metals, nonmetals and metalloids • Hydrogen is different than the others Most elements are found in combination with other elements 5/24/2017 APHY101 13