The Quantum Model of the Atom
... Heisenberg Uncertainty Principle: it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
... Heisenberg Uncertainty Principle: it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
Quantum Field Theory - Institut für Theoretische Physik
... physics in the guise of critical behavior of statistical systems confined to surfaces. ...
... physics in the guise of critical behavior of statistical systems confined to surfaces. ...
Atomic Theory electron charge: -1.6 X 10-19C
... light were conceived of as continuously divisible quantities. With quantum theory, we have the principle that certain physical quantities can only assume discrete quantities. For example, charge cannot have magnitude less than 1.6 X 10-19C. Amounts of electrical charge, then, are always integral mul ...
... light were conceived of as continuously divisible quantities. With quantum theory, we have the principle that certain physical quantities can only assume discrete quantities. For example, charge cannot have magnitude less than 1.6 X 10-19C. Amounts of electrical charge, then, are always integral mul ...
03-02BohrAtom
... • Did not explain • Spectral fine structure • Brightness of lines • Molecular bonds • Theory was not complete. • But otherwise it generally kicked tuckus ...
... • Did not explain • Spectral fine structure • Brightness of lines • Molecular bonds • Theory was not complete. • But otherwise it generally kicked tuckus ...
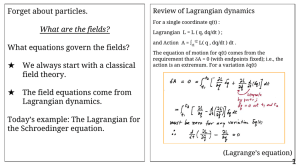
Forget about particles. What equations govern the fields? What are the fields?
... We have, in the Schroedinger picture, [ ψ(x) , ψ♰(x’) ] = δ3(x-x’) , ...
... We have, in the Schroedinger picture, [ ψ(x) , ψ♰(x’) ] = δ3(x-x’) , ...
Computational Complexity and Physics
... The Information Loss Problem: Calculations suggest that Hawking radiation is thermal—uncorrelated with whatever fell in. So, is infalling information lost forever? Would violate the unitarity / reversibility of QM OK then, assume the information somehow gets out! The Xeroxing Problem: How could the ...
... The Information Loss Problem: Calculations suggest that Hawking radiation is thermal—uncorrelated with whatever fell in. So, is infalling information lost forever? Would violate the unitarity / reversibility of QM OK then, assume the information somehow gets out! The Xeroxing Problem: How could the ...
Part One: Light Waves, Photons, and Bohr Theory A. The Wave
... It is impossible to measure both the velocity and position of an e- simultaneously to an arbitrarily high degree of precision. Therefore, cannot view the e- as following a precise trajectory around the nucleus. ...
... It is impossible to measure both the velocity and position of an e- simultaneously to an arbitrarily high degree of precision. Therefore, cannot view the e- as following a precise trajectory around the nucleus. ...
Phys 197 Homework Solution 41A Q3.
... 2s, 2p, 3s, and 3p are filled. If all electrons were are stripped away and one given back, it would find the 3d to be lower in energy that the 4s. The presence of the lower electrons modifies this relationship. Q16. ...
... 2s, 2p, 3s, and 3p are filled. If all electrons were are stripped away and one given back, it would find the 3d to be lower in energy that the 4s. The presence of the lower electrons modifies this relationship. Q16. ...
quantum mechanical model
... Pauli Exclusion Principle: Electrons cannot have the same four quantum numbers within the same atom. Shell: A set of electrons with the same principal quantum number (n). Subshell: A set of electrons with the same azimuthal quantum number (l). ...
... Pauli Exclusion Principle: Electrons cannot have the same four quantum numbers within the same atom. Shell: A set of electrons with the same principal quantum number (n). Subshell: A set of electrons with the same azimuthal quantum number (l). ...
Quantum Number Worksheet - SCH4U-SCHS
... b) List the four quantum numbers for the valence electrons in Ca. Ca has 2 valence electrons in the 4s subshell = 2 sets of quantum numbers ...
... b) List the four quantum numbers for the valence electrons in Ca. Ca has 2 valence electrons in the 4s subshell = 2 sets of quantum numbers ...
Exam Results - University of Wisconsin–Madison
... • to photon emission or absorption which may be represented by a simple diagram - a Feynman studied the idea that all QED processes reduce Feynman diagram. Emission of a photon ...
... • to photon emission or absorption which may be represented by a simple diagram - a Feynman studied the idea that all QED processes reduce Feynman diagram. Emission of a photon ...
Views on Atomic Stru..
... • Each of these orbitals is a different region of space and a different shape •All the ‘l’ quantum values represent different subshells •When n = 1, there is only 1 “l” value meaning there is only one subshell in the first energy level; when n= 2; there are 2 values for ‘l’ indicating two subshells ...
... • Each of these orbitals is a different region of space and a different shape •All the ‘l’ quantum values represent different subshells •When n = 1, there is only 1 “l” value meaning there is only one subshell in the first energy level; when n= 2; there are 2 values for ‘l’ indicating two subshells ...
4.1 and 4.2 notes.pptx
... The electron wasn’t the ONLY sub particle discovered… _____________________________ discovered the proton using the SAME cathode ray tube experiment as___________________________. Each proton is _______________ times more massive than the electron ________________________ discovered the neutron 46 y ...
... The electron wasn’t the ONLY sub particle discovered… _____________________________ discovered the proton using the SAME cathode ray tube experiment as___________________________. Each proton is _______________ times more massive than the electron ________________________ discovered the neutron 46 y ...
Word
... 11) It is harder to see interference with buckyballs than electrons because buckyballs: a) are neutral and harder to accelerate b) are bigger and need bigger slits c) have smaller wavelengths d) have bigger wavelengths 12) Suppose you want to show your wave-like nature with diffraction as you walk t ...
... 11) It is harder to see interference with buckyballs than electrons because buckyballs: a) are neutral and harder to accelerate b) are bigger and need bigger slits c) have smaller wavelengths d) have bigger wavelengths 12) Suppose you want to show your wave-like nature with diffraction as you walk t ...
Prelab notes
... Matter Waves • If energy has dual nature, why not matter? • De Broglie thought so. – Matter Waves – the wavelike behavior of waves. – Didn’t stand without experimental proof ...
... Matter Waves • If energy has dual nature, why not matter? • De Broglie thought so. – Matter Waves – the wavelike behavior of waves. – Didn’t stand without experimental proof ...
Electron Configuration
... Matter Waves • If energy has dual nature, why not matter? • De Broglie thought so. – Matter Waves – the wavelike behavior of waves. – Didn’t stand without experimental proof ...
... Matter Waves • If energy has dual nature, why not matter? • De Broglie thought so. – Matter Waves – the wavelike behavior of waves. – Didn’t stand without experimental proof ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.