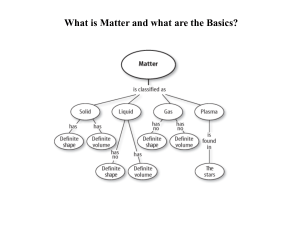
Atomic Theory and the Periodic Table Video Questions
... Which has the smallest mass, a proton, a neutron, or an electron? Who is the Danish physicist who came up with the planetary model of the atom? Where are the electrons found? Specifically which particle distinguishes one atom from another? Who proposed the concept of the atomic number? What do we ge ...
... Which has the smallest mass, a proton, a neutron, or an electron? Who is the Danish physicist who came up with the planetary model of the atom? Where are the electrons found? Specifically which particle distinguishes one atom from another? Who proposed the concept of the atomic number? What do we ge ...
Vocab: 1. Atamos 2. Indivisible 3. Indestructible 4. Atom 5. Electron 6
... 9. Chadwick- discovered the neutron -neutral particle -found in the nucleus 10. Ernest Rutherford -Gold Foil Experiment -Used alpha particles -Discovered the nucleus of the atom -Knew that it was positively charged -reasons- the results of his experiment a. most particles went through the foil b. so ...
... 9. Chadwick- discovered the neutron -neutral particle -found in the nucleus 10. Ernest Rutherford -Gold Foil Experiment -Used alpha particles -Discovered the nucleus of the atom -Knew that it was positively charged -reasons- the results of his experiment a. most particles went through the foil b. so ...
Chapter 2 - Speedway High School
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
Atoms - eChalk
... elements in exactly the same proportions by mass regardless of the size of the sample or the source of the compound. • 3) The law of multiple proportions- if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combines with a cert ...
... elements in exactly the same proportions by mass regardless of the size of the sample or the source of the compound. • 3) The law of multiple proportions- if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combines with a cert ...
The Periodic Table - Harlan Independent Schools
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called ...
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called ...
Unit 2: Atomic Structure and Nuclear Chemistry
... In this unit students will describe how the arrangement of elements in the periodic table and their electron configurations are related. They will describe how the location of an element in the periodic table can be used to predict the properties of that element. Expected learning outcomes: 1. Deter ...
... In this unit students will describe how the arrangement of elements in the periodic table and their electron configurations are related. They will describe how the location of an element in the periodic table can be used to predict the properties of that element. Expected learning outcomes: 1. Deter ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
Unit 2 Practice Exam exam_2p_08_matter
... d. relative sharing of electrons between atoms depends upon their electronegativity. 43. The elements of group 1A of the periodic table are called the a. halogens b. alkaline earth metals c. alkali metals d. noble gases 44. The electrons that are most responsible for an atom’s chemical behavior are ...
... d. relative sharing of electrons between atoms depends upon their electronegativity. 43. The elements of group 1A of the periodic table are called the a. halogens b. alkaline earth metals c. alkali metals d. noble gases 44. The electrons that are most responsible for an atom’s chemical behavior are ...
General Chemistry - Review for final exam: (Make sure you bring
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
Chemistry I Honors – Semester Exam Review – Fall 2000
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
- Chapter 7 Chapter 7 - Periodic Properties of the Elements
... due to increasing Zeff which draws the electrons closer to the nucleus causing the atom to decrease in size. ...
... due to increasing Zeff which draws the electrons closer to the nucleus causing the atom to decrease in size. ...
Test 1
... Atoms contain the same number of protons and electrons. The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an ele ...
... Atoms contain the same number of protons and electrons. The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an ele ...
Name Period _____ Chemistry Review
... ____ 14. A(n) pure substance is made of only one kind of matter and has definite properties. _________________________ ____ 15. A substance that undergoes a chemical change is still the same substance after the change. _________________________ ____ 16. A(n) mixture is made of two or more substances ...
... ____ 14. A(n) pure substance is made of only one kind of matter and has definite properties. _________________________ ____ 15. A substance that undergoes a chemical change is still the same substance after the change. _________________________ ____ 16. A(n) mixture is made of two or more substances ...
Introduction to Atomic Theory
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in single whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in single whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
Chapter 4 notes outline
... number of neutrons Elements can have several isotopes 4.3 Modern Atomic Theory Bohr’s Model of the Atom Better description of electrons Electrons orbit around nucleus in energy levels like planets 1st Level = holds up to 2 electrons 2nd Level = holds up to 8 electrons Electrons can move to d ...
... number of neutrons Elements can have several isotopes 4.3 Modern Atomic Theory Bohr’s Model of the Atom Better description of electrons Electrons orbit around nucleus in energy levels like planets 1st Level = holds up to 2 electrons 2nd Level = holds up to 8 electrons Electrons can move to d ...
- Chapter 7 Chapter 7 - Periodic Properties of the Elements
... due to increasing Zeff which draws the electrons closer to the nucleus causing the atom to decrease in size. ...
... due to increasing Zeff which draws the electrons closer to the nucleus causing the atom to decrease in size. ...
File
... • carbon 14 is radioactive but has similar chemical properties of carbon 12 • mass number – the sum of the number of protons and neutrons in an atom • radiation • Fig. 2.19, pg. 59 • atoms at brainpop.com ...
... • carbon 14 is radioactive but has similar chemical properties of carbon 12 • mass number – the sum of the number of protons and neutrons in an atom • radiation • Fig. 2.19, pg. 59 • atoms at brainpop.com ...
Chapter 21 Powerpoint: Nuclear Chemistry
... After 10 half-lives sample considered nonradioactive because it approaches the level of background radiation. Because the amount never reaches zero, radioactive waste disposal and storage causes ...
... After 10 half-lives sample considered nonradioactive because it approaches the level of background radiation. Because the amount never reaches zero, radioactive waste disposal and storage causes ...
ionization energies
... • A direct indication of the arrangement of electrons about a nucleus is given by the ionization energies of the atom • Ionization energy (IE) is the minimum energy needed to remove an electron (form a cation) completely from a gaseous atom • Ionizations are successive. • As you remove one electron, ...
... • A direct indication of the arrangement of electrons about a nucleus is given by the ionization energies of the atom • Ionization energy (IE) is the minimum energy needed to remove an electron (form a cation) completely from a gaseous atom • Ionizations are successive. • As you remove one electron, ...
Periodic Trends & the Periodic Table
... • All transition elements are metals. • Many transition metals can have more than one charge ...
... • All transition elements are metals. • Many transition metals can have more than one charge ...
Parts of the Atom
... Can not change for an element All atoms are neutral, so Z equals the # of electrons For an ion – the number of electrons may differ ...
... Can not change for an element All atoms are neutral, so Z equals the # of electrons For an ion – the number of electrons may differ ...
Matter and the Periodic Table
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
Chemical Bonds
... made up of tiny particles called atoms can exist in the form of elements and compounds Copper, iron, and lead are elements that can exist by themselves. ...
... made up of tiny particles called atoms can exist in the form of elements and compounds Copper, iron, and lead are elements that can exist by themselves. ...