Honors Midterm Review – 2015-16
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
FIREWORKS EMC summary notes
... The most recently discovered elements have been made by scientists. These elements are found after uranium at the bottom of the Periodic Table. ...
... The most recently discovered elements have been made by scientists. These elements are found after uranium at the bottom of the Periodic Table. ...
•What makes up an atom? Draw an atom
... • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
... • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
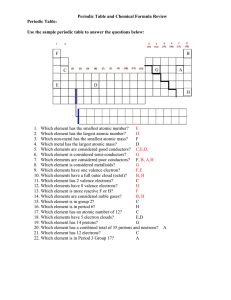
Periodic Table Review Key
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
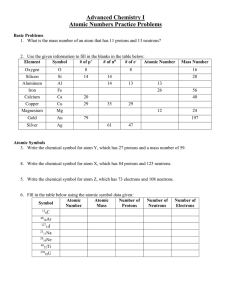
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
5 - BrainMass
... 5.72) Using the values from Thermodynamics Quantities for Selected Substances at 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. F ...
... 5.72) Using the values from Thermodynamics Quantities for Selected Substances at 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. F ...
Science Olympiad
... ______ 6. Ions with the electronic structure 1s2 2s2 2p6 3s2 3p6 would not be present in which aqueous solution? (A) NaF(aq) (B) NaCl(aq) (C) KBr(aq) (D) CaI2(aq) (E) ScBr3 ______ 7. In moving from left to right across a period in the periodic table of the elements (A) ionization energy decreases du ...
... ______ 6. Ions with the electronic structure 1s2 2s2 2p6 3s2 3p6 would not be present in which aqueous solution? (A) NaF(aq) (B) NaCl(aq) (C) KBr(aq) (D) CaI2(aq) (E) ScBr3 ______ 7. In moving from left to right across a period in the periodic table of the elements (A) ionization energy decreases du ...
Atoms - Science with Mrs. Schulte
... Atomic mass The average mass of all the isotopes (different types) of an element ...
... Atomic mass The average mass of all the isotopes (different types) of an element ...
10-2 Intensive Chemistry Review for Chapters 3
... 16. You are NOT responsible for knowing or using the equation on p. 95 for the energy of electrons. 17. What is meant by the statement that each electron has a unique 4-part quantum address around a nucleus? (You do NOT need to memorize table 3-4 on p. 97) 18. The principle quantum number (n level) ...
... 16. You are NOT responsible for knowing or using the equation on p. 95 for the energy of electrons. 17. What is meant by the statement that each electron has a unique 4-part quantum address around a nucleus? (You do NOT need to memorize table 3-4 on p. 97) 18. The principle quantum number (n level) ...
Unit 2 Notes - School City of Hobart
... Nuclear reactions involve changes in atomic nuclei to generate energy. Nuclear Chemistry is the study of those reactions, with an emphasis on their uses in chemistry and their effects on biological systems 21.1 Radioactivity • Nucleon is simply another name for particles in the nucleus (proton/neutr ...
... Nuclear reactions involve changes in atomic nuclei to generate energy. Nuclear Chemistry is the study of those reactions, with an emphasis on their uses in chemistry and their effects on biological systems 21.1 Radioactivity • Nucleon is simply another name for particles in the nucleus (proton/neutr ...
Intro to Element Note Answers
... Alchemists were early chemists whose mean focus was … to turn Pb to Au (change one element into another) Elements are … the building blocks of matter, basic substances that can’t be broken down by ordinary chemical changes ...
... Alchemists were early chemists whose mean focus was … to turn Pb to Au (change one element into another) Elements are … the building blocks of matter, basic substances that can’t be broken down by ordinary chemical changes ...
Notes
... in energy levels (shells). The first energy level may contain up to 2 electrons, the second energy level up to 8 electrons and the third up to 8 electrons. The electron arrangement of an atom can be shown clearly using a target picture e.g. 1st energy level 2nd energy level ...
... in energy levels (shells). The first energy level may contain up to 2 electrons, the second energy level up to 8 electrons and the third up to 8 electrons. The electron arrangement of an atom can be shown clearly using a target picture e.g. 1st energy level 2nd energy level ...
Atomic History notes
... Antoine Lavoiser: A French chemist who in the 1770’s gave the 1st experimental evidence for the law of conservation of matter law of conservation of matter-matter can neither be created or destroyed. Joseph Proust: A French chemist who in 1799 worked on the problem of determining the composition of ...
... Antoine Lavoiser: A French chemist who in the 1770’s gave the 1st experimental evidence for the law of conservation of matter law of conservation of matter-matter can neither be created or destroyed. Joseph Proust: A French chemist who in 1799 worked on the problem of determining the composition of ...
Fire Up Your Atoms!!
... Today’s Agenda: 1. complete Atoms 1 (as group) 2. Review 3. Drawing Atomic Structure I - 3 -complete for HW Study for quiz on Friday--atoms ...
... Today’s Agenda: 1. complete Atoms 1 (as group) 2. Review 3. Drawing Atomic Structure I - 3 -complete for HW Study for quiz on Friday--atoms ...
Unit 1 Atom
... when an x-ray photon transfers its energy to an orbital electron and ejects it from its shell. ...
... when an x-ray photon transfers its energy to an orbital electron and ejects it from its shell. ...
Unit Description - Honors Chemistry
... Use the Aufbau Principle, the Pauli Exclusion Principle and Hund’s Rule to write the electron configurations and orbital diagrams of the elements (5.3) Relate valence electrons to Lewis (electron dot) structures (5.3) Describe the ground-state arrangement of electrons in atoms of any element u ...
... Use the Aufbau Principle, the Pauli Exclusion Principle and Hund’s Rule to write the electron configurations and orbital diagrams of the elements (5.3) Relate valence electrons to Lewis (electron dot) structures (5.3) Describe the ground-state arrangement of electrons in atoms of any element u ...
The Periodic Table: Trends
... acknowledging its orderliness. There are exceptions to trends at times. To help you to focus on the trends, try to look first from peak to peak or from valley to valley. If the charts on the following pages were roller coaster rides, which elements are found at each of the highest points? Is there a ...
... acknowledging its orderliness. There are exceptions to trends at times. To help you to focus on the trends, try to look first from peak to peak or from valley to valley. If the charts on the following pages were roller coaster rides, which elements are found at each of the highest points? Is there a ...
File - Mrs. Riggs Online
... Electrons arranged in concentric layers that surround the nucleus called electron shells/energy levels/clouds/orbitals: ...
... Electrons arranged in concentric layers that surround the nucleus called electron shells/energy levels/clouds/orbitals: ...
Atom Building blocks of matter Proton Sub
... Electron found in outermost shell of an atom; determines atoms chemical properties ...
... Electron found in outermost shell of an atom; determines atoms chemical properties ...
Naming Ionic Compounds
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
Unit 4 – Atomic Structure Study Guide
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
CHEM 1305 - HCC Learning Web
... 21a. Element X has natural isotopes; X-63 (62.940amu) and X-65 (64.928amu). Calculate the atomic mass of element X given the abundance of X-63 is 69.17% b. Which element corresponds to each of the following electron configuration? i. 1S2 2S2 2P5 ii. 1S2 2S2 2P6 3S2 3P6 iii 1S2 2S2 2P6 3S2 3P6 4S2 3d ...
... 21a. Element X has natural isotopes; X-63 (62.940amu) and X-65 (64.928amu). Calculate the atomic mass of element X given the abundance of X-63 is 69.17% b. Which element corresponds to each of the following electron configuration? i. 1S2 2S2 2P5 ii. 1S2 2S2 2P6 3S2 3P6 iii 1S2 2S2 2P6 3S2 3P6 4S2 3d ...