Name: Date: ______ Period: _____ Chemistry 1st semester final
... 40. What type of elements is found in the lower left hand part of the periodic table?metals 41. Where are metalloids found on the periodic table?Along the dark (or red) stair case line 42. From which orbital in a lithium atom is an electron transferred to form Li ion? Looses electrons from 2s 43. Wh ...
... 40. What type of elements is found in the lower left hand part of the periodic table?metals 41. Where are metalloids found on the periodic table?Along the dark (or red) stair case line 42. From which orbital in a lithium atom is an electron transferred to form Li ion? Looses electrons from 2s 43. Wh ...
Chapter 4 Atomic Structure
... regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must also be positive particles in the atom to balance the negative charge of the electrons c) Electrons have very little mass, therefore atoms must contain other p ...
... regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must also be positive particles in the atom to balance the negative charge of the electrons c) Electrons have very little mass, therefore atoms must contain other p ...
How Atoms Differ (Section 4.3) part 1
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
AP CHEMISTRY SUMMER ASSIGNMENT AP Chemistry is a
... 11. Name an element that has similar properties to sodium. __________ 12. State a property of cobalt._______________________________________ 13. Cobalt has a total of ____________ protons. 14. What is the number of protons and neutrons in carbon-14? _____ 15. How many valence electrons are in silico ...
... 11. Name an element that has similar properties to sodium. __________ 12. State a property of cobalt._______________________________________ 13. Cobalt has a total of ____________ protons. 14. What is the number of protons and neutrons in carbon-14? _____ 15. How many valence electrons are in silico ...
Class 9 CBSE Test paper Solved Chapter 3: Structure of...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
Atoms - Sackville School
... This is a diagram of a CD. The diameter of its hole is one-eighth of the diameter of the ...
... This is a diagram of a CD. The diameter of its hole is one-eighth of the diameter of the ...
Protons, Valence Electrons, and the Periodic Table
... • Atoms are the simplest form of an element that cannot be broken down chemically. • They maintain the element’s properties. • Each element is an atom that has the same number of protons – – i.e. the same atomic number on the periodic table of elements. ...
... • Atoms are the simplest form of an element that cannot be broken down chemically. • They maintain the element’s properties. • Each element is an atom that has the same number of protons – – i.e. the same atomic number on the periodic table of elements. ...
Atoms, Molecules and Ions 2
... Now Rutherford’s model explained almost everything that was known about an atom, however it didn’t explain the discrepancy in mass of atoms It was known that hydrogen contained 1 proton and that helium contained two. Therefore the mass of helium should be twice that of hydrogen, however it was not. ...
... Now Rutherford’s model explained almost everything that was known about an atom, however it didn’t explain the discrepancy in mass of atoms It was known that hydrogen contained 1 proton and that helium contained two. Therefore the mass of helium should be twice that of hydrogen, however it was not. ...
Atomic Worksheet
... Where would you find a proton in an atom? ___________________________________ What is the charge of an electron?__________ Where would you find an electron in an atom?_________________________________ What is the charge of a neutron?___________ Where would you find a neutron in an atom? ____________ ...
... Where would you find a proton in an atom? ___________________________________ What is the charge of an electron?__________ Where would you find an electron in an atom?_________________________________ What is the charge of a neutron?___________ Where would you find a neutron in an atom? ____________ ...
Structure of the Atom - Effingham County Schools
... Most particles passed through. So, atoms are mostly empty. Some positive -particles deflected or bounced back! Thus, a “nucleus” is positive & holds most of an atom’s mass. ...
... Most particles passed through. So, atoms are mostly empty. Some positive -particles deflected or bounced back! Thus, a “nucleus” is positive & holds most of an atom’s mass. ...
SNC1D Periodic Table and Atomic Structure Package
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
File - Mr. Henshaw`s Lab
... A. Atoms are made of protons, neutrons, and electrons. B . All atoms of a given element are identical C . Atoms are neither created nor destroyed in chemical ...
... A. Atoms are made of protons, neutrons, and electrons. B . All atoms of a given element are identical C . Atoms are neither created nor destroyed in chemical ...
Nuclear Fusion and Fission
... Nuclear fission was first discovered by two German scientists, Fritz Strassman and Otto Hahn, in the 1930s. They began their work by bombarding uranium with neutrons, hoping to create larger elements. Instead, they were very surprised to find Ba-141, a much smaller element. They immediately contacte ...
... Nuclear fission was first discovered by two German scientists, Fritz Strassman and Otto Hahn, in the 1930s. They began their work by bombarding uranium with neutrons, hoping to create larger elements. Instead, they were very surprised to find Ba-141, a much smaller element. They immediately contacte ...
Atom Internet Scavenger Hunt
... as you move to the right in a Period. If it is true explain why. If it is false explain why. (Evaluating) This statement is true. As you move down a Group you add an energy level. The reason the atoms get smaller is due to the increase in the atomic number. Each element has one more proton than its ...
... as you move to the right in a Period. If it is true explain why. If it is false explain why. (Evaluating) This statement is true. As you move down a Group you add an energy level. The reason the atoms get smaller is due to the increase in the atomic number. Each element has one more proton than its ...
Atoms, Molecules and Ions
... • isotopes--atoms having the same atomic number (# of p+) but a different number of neutrons • most elements have at least two stable isotopes, there are very few with only one stable isotope (Al, F, P) • Hydrogen’s isotopes are so important they have special names: 0 neutrons hydrogen 1 neutron ...
... • isotopes--atoms having the same atomic number (# of p+) but a different number of neutrons • most elements have at least two stable isotopes, there are very few with only one stable isotope (Al, F, P) • Hydrogen’s isotopes are so important they have special names: 0 neutrons hydrogen 1 neutron ...
atom
... • Suggested that the electrons orbiting the nucleus of atoms can only have certain discrete energies. • Electrons lose/gain energy in order to move from one energy level to another. • The emission of light (photon) occurs when an electron moves from a higher to a lower energy orbit (emission spectra ...
... • Suggested that the electrons orbiting the nucleus of atoms can only have certain discrete energies. • Electrons lose/gain energy in order to move from one energy level to another. • The emission of light (photon) occurs when an electron moves from a higher to a lower energy orbit (emission spectra ...
Atomic Theory, and the Periodic Table
... 10. In 1926, Erwin Schrodinger proposed the electron cloud model. These regions of space containing the electrons are called electron clouds. ...
... 10. In 1926, Erwin Schrodinger proposed the electron cloud model. These regions of space containing the electrons are called electron clouds. ...
04 Atom-Review-Worksheet
... atoms that have the same number of protons but different numbers of neutrons weighted average mass of the atoms in a naturally occurring sample of an element equals the number of neutrons plus the number of protons in an atom 1/12 the mass of a carbon-12 atom the number of protons in the nucleus of ...
... atoms that have the same number of protons but different numbers of neutrons weighted average mass of the atoms in a naturally occurring sample of an element equals the number of neutrons plus the number of protons in an atom 1/12 the mass of a carbon-12 atom the number of protons in the nucleus of ...
The Atom Powerpoint 10-16-13
... the orbit of the more complex sublevels is more than that of more simple orbits, a sublevel will not completely fill before the next higher one begins receiving electron. ...
... the orbit of the more complex sublevels is more than that of more simple orbits, a sublevel will not completely fill before the next higher one begins receiving electron. ...
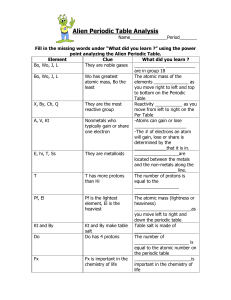
Alien Periodic Table Analysis
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...