Final Exam Practice Problems Set 2
... When 50.0 mL of 0.400 M Ca(NO3)2 is added to 50.0 mL of 0.800 M NaF, CaF2 precipitates, as shown in the net ionic equation below. The initial temperature of both solutions is 21.0 ˚C. Assuming that the reaction goes to completion, and that the resulting solution has a mass of 100.00 g and a specific ...
... When 50.0 mL of 0.400 M Ca(NO3)2 is added to 50.0 mL of 0.800 M NaF, CaF2 precipitates, as shown in the net ionic equation below. The initial temperature of both solutions is 21.0 ˚C. Assuming that the reaction goes to completion, and that the resulting solution has a mass of 100.00 g and a specific ...
Group 2 Elements
... •understand reasons for the trend in ionisation energy down Group 2 •understand reasons for the trend in reactivity of the Group 2 elements down the group •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours ...
... •understand reasons for the trend in ionisation energy down Group 2 •understand reasons for the trend in reactivity of the Group 2 elements down the group •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours ...
Chapt2
... noble gases ............................. group 0 (18): He, Ne, Ar, ... lanthanide elements................ # 58 - 71 (1st row at bottom) actinide elements .................... # 90 - 103 (2nd row at bottom) ...
... noble gases ............................. group 0 (18): He, Ne, Ar, ... lanthanide elements................ # 58 - 71 (1st row at bottom) actinide elements .................... # 90 - 103 (2nd row at bottom) ...
Atoms: The Building Blocks of Matter
... • Compound: a substance that consists of two or more different types of elements. • Molecule: a substance that consists of two or more atoms. ...
... • Compound: a substance that consists of two or more different types of elements. • Molecule: a substance that consists of two or more atoms. ...
Fall 2015 Review-2
... c. all have ions with a 1 charge b. all form ions with a negative charge d. lose electrons when they form ions ____ 38. Which of the following statements correctly compares the relative size of an ion to its neutral atom? a. The radius of an anion is greater than the radius of its neutral atom. b. T ...
... c. all have ions with a 1 charge b. all form ions with a negative charge d. lose electrons when they form ions ____ 38. Which of the following statements correctly compares the relative size of an ion to its neutral atom? a. The radius of an anion is greater than the radius of its neutral atom. b. T ...
atomic
... • the sum of the number of protons and the number of neutrons in the nucleus of an atom. • # of neutrons = Neutrons mass # - atomic # ...
... • the sum of the number of protons and the number of neutrons in the nucleus of an atom. • # of neutrons = Neutrons mass # - atomic # ...
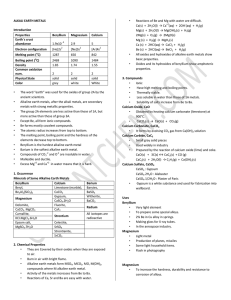
ALKALI EARTH METALS Introduction Properties Beryllium
... The word “earth” was used for the oxides of group 2A by the ancient scientists. Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form io ...
... The word “earth” was used for the oxides of group 2A by the ancient scientists. Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form io ...
different types of atoms
... Chemistry: Atomic Structure The three subatomic particles are: Proton – Positive charge Electron – Negative charge Neutron – No charge or neutral ...
... Chemistry: Atomic Structure The three subatomic particles are: Proton – Positive charge Electron – Negative charge Neutron – No charge or neutral ...
Historical Model of the Atom
... •Dalton’s law of multiple proportions: When two elements form different compounds, the mass ratio of the elements in one compound is related to the mass ratio in the other by a small whole number. ...
... •Dalton’s law of multiple proportions: When two elements form different compounds, the mass ratio of the elements in one compound is related to the mass ratio in the other by a small whole number. ...
Lecture Notes Part 2 - Dr. Samples` Chemistry Classes
... From this experiment, Millikan obtained the actual charge on an electron, 1.60x10-19 C (Don’t memorize!). From this charge and the charge/mass ratio of an electron which JJ Thomson had measured, the exact mass of an electron was calculated to be 9.10x10-28 g (Don’t memorize!). Since electrons have a ...
... From this experiment, Millikan obtained the actual charge on an electron, 1.60x10-19 C (Don’t memorize!). From this charge and the charge/mass ratio of an electron which JJ Thomson had measured, the exact mass of an electron was calculated to be 9.10x10-28 g (Don’t memorize!). Since electrons have a ...
Unit 3 Rev Pckt - Old Saybrook Public Schools
... 9. All atoms of the same element have the 10. The electron cloud is charged. I 1. The part of the atom which is very small and very dense: 12. The electron has a mass a.m.u. 14. One of sulfur (S) atoms has a mass of 32 grams. 15, Many elements on the periodic table have atomic masses which are not c ...
... 9. All atoms of the same element have the 10. The electron cloud is charged. I 1. The part of the atom which is very small and very dense: 12. The electron has a mass a.m.u. 14. One of sulfur (S) atoms has a mass of 32 grams. 15, Many elements on the periodic table have atomic masses which are not c ...
Electron Configuration
... Arranged by increasing atomic mass with similar elements in columns ...
... Arranged by increasing atomic mass with similar elements in columns ...
Atom (A) or Ion
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
Atomic Theory PPT
... Niels Bohr Niels Bohr stated that electrons move in different orbits, or energy levels, around the nucleus like planets orbit the sun. Each energy level is located a specific distance from the nucleus and contains a certain number of electrons. ...
... Niels Bohr Niels Bohr stated that electrons move in different orbits, or energy levels, around the nucleus like planets orbit the sun. Each energy level is located a specific distance from the nucleus and contains a certain number of electrons. ...
Atom (A) or Ion (I)
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
Chem Final Study Guide Energy How much heat energy must be
... properties with both metals and nonmetals. 50) Looking at the periodic table, list 4 atomic numbers that represent elements with similar chemical properties. Why did you choose those numbers? a) Any 4 numbers within the same family/group. Ex: 4, 20, 38, 56 Chemical Reactions 51) Predict the products ...
... properties with both metals and nonmetals. 50) Looking at the periodic table, list 4 atomic numbers that represent elements with similar chemical properties. Why did you choose those numbers? a) Any 4 numbers within the same family/group. Ex: 4, 20, 38, 56 Chemical Reactions 51) Predict the products ...
Chemistry for Biology
... Medical uses for radioactive isotopes A PET scan is one of the many medical uses for radioactive isotopes PET, short for positronemission tomography, can detect intense chemical activity in the body. Brightly colored areas indicate elevated levels of radioactively-labeled glucose and high metabolic ...
... Medical uses for radioactive isotopes A PET scan is one of the many medical uses for radioactive isotopes PET, short for positronemission tomography, can detect intense chemical activity in the body. Brightly colored areas indicate elevated levels of radioactively-labeled glucose and high metabolic ...
Atom (A) or Ion (I)
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
... 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny indivisible particles called atoms. B) Atoms are made of smaller ...
word-doc Practice for the final exam!
... atomic radius __________; (2) the electron affinity becomes __________ negative; and (3) the first ionization energy ___________. a. decreases, decreasingly, increases b. increases, increasingly, decreases c. increases, increasingly, increases d. decreases, increasingly, increases e. decreases, decr ...
... atomic radius __________; (2) the electron affinity becomes __________ negative; and (3) the first ionization energy ___________. a. decreases, decreasingly, increases b. increases, increasingly, decreases c. increases, increasingly, increases d. decreases, increasingly, increases e. decreases, decr ...
Answer = 1.81 x 10 24 molecules
... • Chemists also agreed that elements could combine to form compounds that have different physical and chemical properties than those of the elements used to form them Ex. NaCl has different physical and chemical properties than chlorine (Cl) and Sodium (Na) • There was controversy over whether ele ...
... • Chemists also agreed that elements could combine to form compounds that have different physical and chemical properties than those of the elements used to form them Ex. NaCl has different physical and chemical properties than chlorine (Cl) and Sodium (Na) • There was controversy over whether ele ...
Chapter 2: Matter is Made up of Atoms
... chocolate chip cookie dough (see Fig 2.8 p 63 of text) • 1911-Rutherford’s Gold Foil Experiment • Shot “alpha Particles” (helium nuclei) at gold foil. • Hypothesis: they would pass through unaffected. • Data: most did pass through – Some were deflected – Others bounced straight back! ...
... chocolate chip cookie dough (see Fig 2.8 p 63 of text) • 1911-Rutherford’s Gold Foil Experiment • Shot “alpha Particles” (helium nuclei) at gold foil. • Hypothesis: they would pass through unaffected. • Data: most did pass through – Some were deflected – Others bounced straight back! ...
Atomic Theory
... Discoveries of subatomic particles and related advances During the 1800s, there was a gradual acceptance of a “billiard ball” model of an indivisible atom ...
... Discoveries of subatomic particles and related advances During the 1800s, there was a gradual acceptance of a “billiard ball” model of an indivisible atom ...
Topic_7_atomic_and_nuclear_energy_IB_problems
... This question is about the nuclear structure of the atom and atomic energy levels. When the electron was first discovered it led to the idea that an atom consists of a lump of positive charge in which the electrons are embedded. In 1912 Geiger and Marsden carried out an experiment to test the validi ...
... This question is about the nuclear structure of the atom and atomic energy levels. When the electron was first discovered it led to the idea that an atom consists of a lump of positive charge in which the electrons are embedded. In 1912 Geiger and Marsden carried out an experiment to test the validi ...
Atomic Structure
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...