ch. 4 atomic structure
... An Electron Cloud is a visual model of the most likely locations for the electrons in an atom. Scientists use this to describe how electrons move around the nucleus. Think of an airplane propeller, when the plane isn’t moving you can count the blades present, however, when it starts to move the blad ...
... An Electron Cloud is a visual model of the most likely locations for the electrons in an atom. Scientists use this to describe how electrons move around the nucleus. Think of an airplane propeller, when the plane isn’t moving you can count the blades present, however, when it starts to move the blad ...
atomic structure revision and questions 08
... ATOMIC STRUCTURE REVISION AND QUESTIONS 2015 Key words: element nucleus molecule neutron atom proton mass number ...
... ATOMIC STRUCTURE REVISION AND QUESTIONS 2015 Key words: element nucleus molecule neutron atom proton mass number ...
Atomic Structure
... into gold foil, most pass straight through while a few are violently deflected. • This implies a dense, positively charged central region containing most of the atomic mass and that the atom is mostly space. ...
... into gold foil, most pass straight through while a few are violently deflected. • This implies a dense, positively charged central region containing most of the atomic mass and that the atom is mostly space. ...
Chapter 10 PowerPoint
... slightest bit more mass. Protons and electrons may differ in size but their charges cancel each other out. If charges are unequal then you will have an ion. -1 electron then positive ion. +1 electron then negative ion. The diameter of the nucleus is 1/100,000 the diameter of the atom. ...
... slightest bit more mass. Protons and electrons may differ in size but their charges cancel each other out. If charges are unequal then you will have an ion. -1 electron then positive ion. +1 electron then negative ion. The diameter of the nucleus is 1/100,000 the diameter of the atom. ...
CHMR_AYS_U4AlienPeriodicTableAnalysis_V01
... _____________ as you move from right to left and top to bottom on the Periodic Table. Reactivity ______________ as you move from left to right on the Periodic Table, except for the ________ ____. Atoms can gain or lose _____________. The # of electrons an atom will gain, lose or share is determined ...
... _____________ as you move from right to left and top to bottom on the Periodic Table. Reactivity ______________ as you move from left to right on the Periodic Table, except for the ________ ____. Atoms can gain or lose _____________. The # of electrons an atom will gain, lose or share is determined ...
Chapter 4 Notes
... • Silver always has 47 protons, so it is unnecessary to write it in the above format. ...
... • Silver always has 47 protons, so it is unnecessary to write it in the above format. ...
Reporting Category 2: Atomic Structure and Nuclear Chemistry
... • Elements are made of very small, indivisible particles called atoms. • All atoms of a given element are identical. • Atoms of a given element are different from those of any other element and have different atomic masses. • In a chemical reaction, atoms of one element can combine with atoms of ano ...
... • Elements are made of very small, indivisible particles called atoms. • All atoms of a given element are identical. • Atoms of a given element are different from those of any other element and have different atomic masses. • In a chemical reaction, atoms of one element can combine with atoms of ano ...
Introductory Chemistry: A Foundation FOURTH EDITION by
... physical properties are in the same column • Columns are called Groups or Families • Rows are called Periods • Each period shows the pattern of properties repeated in the next period ...
... physical properties are in the same column • Columns are called Groups or Families • Rows are called Periods • Each period shows the pattern of properties repeated in the next period ...
Chapter 5 - HCC Learning Web
... DALTON’S ATOMIC THEORY Dalton proposed that: An element is composed of tiny, indivisible, indestructible particles called atoms. All atoms of an element are identical and have the same properties. Atoms of different elements combine to form compounds. Compounds contain atoms in small whole n ...
... DALTON’S ATOMIC THEORY Dalton proposed that: An element is composed of tiny, indivisible, indestructible particles called atoms. All atoms of an element are identical and have the same properties. Atoms of different elements combine to form compounds. Compounds contain atoms in small whole n ...
PRACTICE * Naming and Writing Ionic Compounds
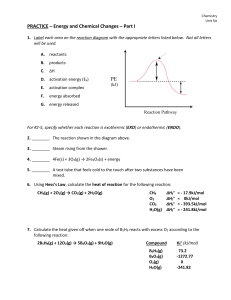
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
word doc (perfect formatting)
... 2) Represents an atom that is a noble gas 3) Represents an atom that is a transition metal 4) Represents an atom of an alkali earth metal Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positive and negative ions held together by electrostatic ...
... 2) Represents an atom that is a noble gas 3) Represents an atom that is a transition metal 4) Represents an atom of an alkali earth metal Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positive and negative ions held together by electrostatic ...
Atoms, Molecules and Ions
... • Dalton’s basic postulates (ideas) were: – All matter is composed of atoms which are indivisible and indestructible. Atoms are considered as the ultimate chemical particles. – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combin ...
... • Dalton’s basic postulates (ideas) were: – All matter is composed of atoms which are indivisible and indestructible. Atoms are considered as the ultimate chemical particles. – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combin ...
Ch. 3 - Chemical Reactions
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
Chapter 16: The Properties of Atoms and the Periodic Table
... • In 1926, Werner Heisinberg, based on quantum mechanics, demonstrated it was impossible to know both the motion and location of an electron at the same time Heisenberg proposed that the electrons form a cloud around the nucleus of an atom. In the electron cloud were regions called orbitals where ...
... • In 1926, Werner Heisinberg, based on quantum mechanics, demonstrated it was impossible to know both the motion and location of an electron at the same time Heisenberg proposed that the electrons form a cloud around the nucleus of an atom. In the electron cloud were regions called orbitals where ...
History Atomic Theory
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
Matter—anything that has mass and occupies space Weight—pull of
... Radiant or electromagnetic energy Travels in waves (e.g., visible light, ultraviolet light, and x-rays) Energy may be converted from one form to another Energy conversion is inefficient Some energy is “lost” as heat (partly unusable energy) ...
... Radiant or electromagnetic energy Travels in waves (e.g., visible light, ultraviolet light, and x-rays) Energy may be converted from one form to another Energy conversion is inefficient Some energy is “lost” as heat (partly unusable energy) ...
Summary 4.1 Studying Atoms
... numbers of protons. Each positive charge in an atom is balanced by a negative charge because atoms are neutral. So the atomic number of an element also equals the number of electrons in an atom. The mass number of an atom is the sum of the protons and neutrons in its nucleus. Therefore, the number o ...
... numbers of protons. Each positive charge in an atom is balanced by a negative charge because atoms are neutral. So the atomic number of an element also equals the number of electrons in an atom. The mass number of an atom is the sum of the protons and neutrons in its nucleus. Therefore, the number o ...
Document
... the alphabet of the universe. 9. In the alphabet of the universe, atoms are like letters and like words. ...
... the alphabet of the universe. 9. In the alphabet of the universe, atoms are like letters and like words. ...
standard 1 - Taylorsville-Paxton
... gold atoms in gold foil. Occasionally, one of the particles bounced back off the gold foil but most went through. What did Rutherford prove? a. b. c. d. ...
... gold atoms in gold foil. Occasionally, one of the particles bounced back off the gold foil but most went through. What did Rutherford prove? a. b. c. d. ...
Elements and Compounds checklist for web
... -‐ Construct a model of an atom. Use any materials that you like. Your model must have a key. Distinguish between physical and chemical properties, using examples. • physical -‐ solid, liquid or gas - ...
... -‐ Construct a model of an atom. Use any materials that you like. Your model must have a key. Distinguish between physical and chemical properties, using examples. • physical -‐ solid, liquid or gas - ...