atomic structure
... because I cannot monitor what else you are doing while I am focused on teaching. If you do not need to be using your technology for a specific portion of the lesson then it must be upside down in the corner of your desk, or turned off and put away if we will no longer use it that day. ...
... because I cannot monitor what else you are doing while I am focused on teaching. If you do not need to be using your technology for a specific portion of the lesson then it must be upside down in the corner of your desk, or turned off and put away if we will no longer use it that day. ...
Modern Atomic Theory: Electron Cloud Model
... electrons travel around nucleus in definite paths. Paths were located certain distances from nucleus. Electrons could jump from level to level. ...
... electrons travel around nucleus in definite paths. Paths were located certain distances from nucleus. Electrons could jump from level to level. ...
Atomic Structure
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
Chapter 3: Atomic Structure
... and # no •“belt of stability” – as atomic number increases, you need more neutrons to keep the atom stable ...
... and # no •“belt of stability” – as atomic number increases, you need more neutrons to keep the atom stable ...
video slide
... Atoms -- differ in number of subatomic particles atomic number -- number of protons mass number -- protons + neutrons atomic mass -- approximated by the mass number (mass number + electrons) ...
... Atoms -- differ in number of subatomic particles atomic number -- number of protons mass number -- protons + neutrons atomic mass -- approximated by the mass number (mass number + electrons) ...
Chapter 4 Packet Chem
... •List the main points of Dalton’s atomic theory and describe his evidence for the existence of atoms. ...
... •List the main points of Dalton’s atomic theory and describe his evidence for the existence of atoms. ...
PRACTICE * Naming and Writing Ionic Compounds
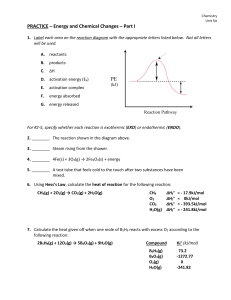
... PRACTICE – Energy and Chemical Changes – Part I 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
... PRACTICE – Energy and Chemical Changes – Part I 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
chapter 2 - Columbia University
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
History of Atomic Theory
... the electrons travel • Electrons in the same shell are approx. the same distance from the ...
... the electrons travel • Electrons in the same shell are approx. the same distance from the ...
Compound Name
... Key Concepts and Vocabulary What’s the difference between a physical and chemical change? Physical changes: examples – change of state, dissolving; Chemical changes (new substance formed): heat or light given off, bubbles/gas produced, colour change, solid precipitate, usually irreversible; Groups a ...
... Key Concepts and Vocabulary What’s the difference between a physical and chemical change? Physical changes: examples – change of state, dissolving; Chemical changes (new substance formed): heat or light given off, bubbles/gas produced, colour change, solid precipitate, usually irreversible; Groups a ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Early Atomic Theory - Columbia University
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
Chapter-2-Human-Chemistry
... Energy “Currency” – Body’s energy carrier Supplier of energy for many of body’s reactions ...
... Energy “Currency” – Body’s energy carrier Supplier of energy for many of body’s reactions ...
200 ways to pass the regents
... 175. Natural polymers include starch, cellulose, and proteins. 176. Synthetic polymers include plastics such as nylon, rayon, and polyester. 177. Unstable atoms that are radioactive are called radioisotopes. (Table N) 178. Radioisotopes can decay by giving off any of the particles/emanations listed ...
... 175. Natural polymers include starch, cellulose, and proteins. 176. Synthetic polymers include plastics such as nylon, rayon, and polyester. 177. Unstable atoms that are radioactive are called radioisotopes. (Table N) 178. Radioisotopes can decay by giving off any of the particles/emanations listed ...
Final Exam Chemistry B2A Mr. Kimball`s Class 2003
... 22. Which of the following is FALSE regarding an electron? a) has a relative charge of -1 b) is abbreviated ec) has a mass of approximately 1 amu d) exists outside the nucleus e) exists in energy levels 23. Which of the following is FALSE regarding the nucleus of an atom? a) a small, low density reg ...
... 22. Which of the following is FALSE regarding an electron? a) has a relative charge of -1 b) is abbreviated ec) has a mass of approximately 1 amu d) exists outside the nucleus e) exists in energy levels 23. Which of the following is FALSE regarding the nucleus of an atom? a) a small, low density reg ...
Concept Reviews Answer sheet Section: matter and Energy 1. a
... 3. Coffee at 38°C has more kinetic energy than coffee at 34°C. Although there is less tea than coffee, the temperature of the tea is greater, so the tea has more average kinetic energy than the coffee. 4. The temperature, and therefore the kinetic energy of the particles in 0.5 L of coffee and 0.25 ...
... 3. Coffee at 38°C has more kinetic energy than coffee at 34°C. Although there is less tea than coffee, the temperature of the tea is greater, so the tea has more average kinetic energy than the coffee. 4. The temperature, and therefore the kinetic energy of the particles in 0.5 L of coffee and 0.25 ...
atomic number = of
... atoms alpha particles should go straight through. • Used gold foil because it could be made atoms ...
... atoms alpha particles should go straight through. • Used gold foil because it could be made atoms ...
Modern Atomic Theory
... • For a set of degenerate orbitals, fill each orbital halfway first before pairing • Electron configurations show how many electrons are in each sublevel of an atom – describes where electrons are. - 1s22s1 is the electron configuration for a ground state Li - 1s22s22p3 is for nitrogen ...
... • For a set of degenerate orbitals, fill each orbital halfway first before pairing • Electron configurations show how many electrons are in each sublevel of an atom – describes where electrons are. - 1s22s1 is the electron configuration for a ground state Li - 1s22s22p3 is for nitrogen ...
Revision Notes chapter 1
... Henry Moseley, a member of Rutherford’s team compared the positive charges of the nuclei of different elements. He found that the charge increases by one unit from element to element in the periodic table. He showed that the sequence of elements in the table is related to the charge of the atoms ...
... Henry Moseley, a member of Rutherford’s team compared the positive charges of the nuclei of different elements. He found that the charge increases by one unit from element to element in the periodic table. He showed that the sequence of elements in the table is related to the charge of the atoms ...
Fall Final Review Honors
... Dalton’s billiard ball model-sphere of uniform density. Thomson’s plum pudding model-negative electrons dispersed in positive atom. Rutherford’s nuclear model-dense, positive nucleus surrounded by negative electrons. Bohr’s planetary model-electrons move in circular orbits in specific energy levels. ...
... Dalton’s billiard ball model-sphere of uniform density. Thomson’s plum pudding model-negative electrons dispersed in positive atom. Rutherford’s nuclear model-dense, positive nucleus surrounded by negative electrons. Bohr’s planetary model-electrons move in circular orbits in specific energy levels. ...