Study Guide: Elements, Compounds, Mixtures Physical Properties
... Can be observed or measured without chemically changing a substance. Can be used to identify an unknown substance (some are more useful for this purpose than others, such as: specific heat, density (mass/volume), melting point, boiling point) Are: malleability, solubility, density, melting & boiling ...
... Can be observed or measured without chemically changing a substance. Can be used to identify an unknown substance (some are more useful for this purpose than others, such as: specific heat, density (mass/volume), melting point, boiling point) Are: malleability, solubility, density, melting & boiling ...
u3ohnotesf2005 - Teach-n-Learn-Chem
... Shorthand Electron Configuration (S.E.C.) To write S.E.C. for an element: 1. Put symbol of noble gas that precedes element in brackets. 2. Continue writing e– config. from that point. ...
... Shorthand Electron Configuration (S.E.C.) To write S.E.C. for an element: 1. Put symbol of noble gas that precedes element in brackets. 2. Continue writing e– config. from that point. ...
5.1 The Development of Atomic Models
... In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. ...
... In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. ...
Lecture 10
... • Atoms make up all the matter around us, but there are only 118 distinct types of atoms (to date). These are called elements. • The elements combine in an infinite # of different ways in order to yield huge variety of substances. • Actually, only 88 of the 118 discovered, are found naturally. Other ...
... • Atoms make up all the matter around us, but there are only 118 distinct types of atoms (to date). These are called elements. • The elements combine in an infinite # of different ways in order to yield huge variety of substances. • Actually, only 88 of the 118 discovered, are found naturally. Other ...
Double-Replacement Reactions - Fort Thomas Independent Schools
... compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually water. • The other compound is often soluble and remains dissolved in solution. ...
... compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually water. • The other compound is often soluble and remains dissolved in solution. ...
Document
... The 1st and 2nd ionization energy for potassium Double the electron affinity for oxygen Double the 1st ionization energy for potassium Ionization energy and electron affinity are not needed for the calculation ...
... The 1st and 2nd ionization energy for potassium Double the electron affinity for oxygen Double the 1st ionization energy for potassium Ionization energy and electron affinity are not needed for the calculation ...
Chemistry Standards and Frameworks
... Most periodic tables have a heavy stepped line running from boron to astatine. Elements to the immediate right and left of this line, excluding the metal aluminum, are semimetals and have properties that are intermediate between metals and nonmetals. Elements further to the left are metals. Those fu ...
... Most periodic tables have a heavy stepped line running from boron to astatine. Elements to the immediate right and left of this line, excluding the metal aluminum, are semimetals and have properties that are intermediate between metals and nonmetals. Elements further to the left are metals. Those fu ...
4 hon chem classifying matter b
... Properties are usually measured by looking at large (~1023) aggregations of atoms or molecules ...
... Properties are usually measured by looking at large (~1023) aggregations of atoms or molecules ...
Chemistry Review
... a change in matter from one form to another without a change in chemical properties attractive force between two oppositely charged ions that result from the transfer of electrons from one atom to another a change that occurs when a substance changes composition by forming one or more new substances ...
... a change in matter from one form to another without a change in chemical properties attractive force between two oppositely charged ions that result from the transfer of electrons from one atom to another a change that occurs when a substance changes composition by forming one or more new substances ...
apchap6 Mast Atomic structure
... They are less effected by the nucleus. lower effective nuclear charge ...
... They are less effected by the nucleus. lower effective nuclear charge ...
7th Science - Carterville CUSD #5
... 13. Atoms of the SAME ELEMENT always have the SAME NUMBER OF ________________. The two ways that atoms of the SAME ELEMENT can be different are if the number of _______________________ changes (then it is called an ___________________) or the number of _________________________ changes (this is call ...
... 13. Atoms of the SAME ELEMENT always have the SAME NUMBER OF ________________. The two ways that atoms of the SAME ELEMENT can be different are if the number of _______________________ changes (then it is called an ___________________) or the number of _________________________ changes (this is call ...
Lesson 13: Nuclear Propulsion Basics
... • Because neutrons are electrically neutral, they are unaffected by Coloumbic or nuclear forces until they reach within 10-15 m of an atomic nucleus – Best particles to use for FISSION ...
... • Because neutrons are electrically neutral, they are unaffected by Coloumbic or nuclear forces until they reach within 10-15 m of an atomic nucleus – Best particles to use for FISSION ...
The Development of Atomic Theory
... of electrons to specific energy levels An electron is never found between these level Electrons jump between energy levels when an atom gains or loses energy The lowest state of energy of an electron is called the ground state If an electron gains energy, it moves to an excited state…gains e ...
... of electrons to specific energy levels An electron is never found between these level Electrons jump between energy levels when an atom gains or loses energy The lowest state of energy of an electron is called the ground state If an electron gains energy, it moves to an excited state…gains e ...
Powerpoint for Scientist Contributions and the Modern Theory of
... • 460 BC - Greek philosopher proposes the existence of the atom • He pounded materials until he made them into smaller and smaller parts • He called them atoma which is Greek for “indivisible”. ...
... • 460 BC - Greek philosopher proposes the existence of the atom • He pounded materials until he made them into smaller and smaller parts • He called them atoma which is Greek for “indivisible”. ...
Chapter 4 Note Guide
... ________________________. For example, oxygen has 3 isotopes: oxygen-16, oxygen-17, and oxygen-18. All three oxygen isotopes can react with hydrogen to form water or combine with iron to form rust. With most elements, ____________________________________________________________________ _____________ ...
... ________________________. For example, oxygen has 3 isotopes: oxygen-16, oxygen-17, and oxygen-18. All three oxygen isotopes can react with hydrogen to form water or combine with iron to form rust. With most elements, ____________________________________________________________________ _____________ ...
Unit A Review Questions
... and are being reduced by the zinc metal being oxidized. This would also account for the colour change in the copper nitrate solution. As the copper ions come out of the solution, the solution becomes a fainter colour. b. The voltage reading is zero because the electrons do not have to travel through ...
... and are being reduced by the zinc metal being oxidized. This would also account for the colour change in the copper nitrate solution. As the copper ions come out of the solution, the solution becomes a fainter colour. b. The voltage reading is zero because the electrons do not have to travel through ...
Atoms - rcasao
... nucleus has a total positive charge +Z·e. • The electrons are negatively charged (-e), so they are attracted to the nucleus and repelled by each other. ...
... nucleus has a total positive charge +Z·e. • The electrons are negatively charged (-e), so they are attracted to the nucleus and repelled by each other. ...
H 2 O
... Temperature, Pressure, And Volume • Volume – Pressure Relationship – At a constant temperature, volume is inversely proportional to pressure ...
... Temperature, Pressure, And Volume • Volume – Pressure Relationship – At a constant temperature, volume is inversely proportional to pressure ...
10_Chemistry homework
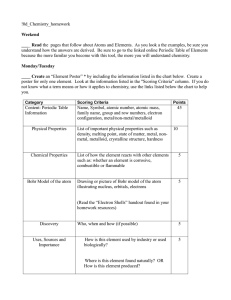
... ____ Create an “Element Poster” * by including the information listed in the chart below. Create a poster for only one element. Look at the information listed in the "Scoring Criteria" column. If you do not know what a term means or how it applies to chemistry, use the links listed below the chart t ...
... ____ Create an “Element Poster” * by including the information listed in the chart below. Create a poster for only one element. Look at the information listed in the "Scoring Criteria" column. If you do not know what a term means or how it applies to chemistry, use the links listed below the chart t ...