Oct 27 day ten
... Rutherford’s contributions to the atomic model Since most of the alpha particles passed through the foil: 1. Most of the atom is empty space Since some of the alpha particles bounced back: 2. Part of the atom is very dense and positive. (He called this the nucleus) ...
... Rutherford’s contributions to the atomic model Since most of the alpha particles passed through the foil: 1. Most of the atom is empty space Since some of the alpha particles bounced back: 2. Part of the atom is very dense and positive. (He called this the nucleus) ...
AP CHEMISTRY - An Incomplete List of Topics
... Example: When Na2CO3(s) is heated, it will decompose to form CO2(g). Solid Na2O will also be formed as the remainder of the original compound. The Na2O(s) will slowly re-absorb CO2(g) from the air and convert back into Na2CO3(s). Other carbonates follow this same pattern, as seen in the reaction of ...
... Example: When Na2CO3(s) is heated, it will decompose to form CO2(g). Solid Na2O will also be formed as the remainder of the original compound. The Na2O(s) will slowly re-absorb CO2(g) from the air and convert back into Na2CO3(s). Other carbonates follow this same pattern, as seen in the reaction of ...
atom
... protons, and neutrons), but the atom is still the smallest body that retains the unique identity of an element Atoms of one element cannot be converted into atoms of another element in a chemical reaction All atoms of an element have the same number of protons and electrons, which determines the che ...
... protons, and neutrons), but the atom is still the smallest body that retains the unique identity of an element Atoms of one element cannot be converted into atoms of another element in a chemical reaction All atoms of an element have the same number of protons and electrons, which determines the che ...
4-1 Introduction to Atoms Directed Reading Questions
... Directions: As you read 4-1, answer the following questions on a separate piece of paper. 1. How is the painting in figure 1 like matter? Like these images, matter is made of tiny parts called atoms. (p. 124) 2. Why have the shape and structure of atoms changed many times? Because atoms are so small ...
... Directions: As you read 4-1, answer the following questions on a separate piece of paper. 1. How is the painting in figure 1 like matter? Like these images, matter is made of tiny parts called atoms. (p. 124) 2. Why have the shape and structure of atoms changed many times? Because atoms are so small ...
AP Ch 07 apchapt7r2
... Emission spectrum because these are the colors it gives off or emits. Called a line spectrum. There are just a few discrete lines showing only certain energies are possible. ...
... Emission spectrum because these are the colors it gives off or emits. Called a line spectrum. There are just a few discrete lines showing only certain energies are possible. ...
Chapter 7 Electron Structure of the Atom
... It takes a little bit of energy to pair up electrons, so single electrons occupy different orbitals of the same energy (Hund’s Rule) ...
... It takes a little bit of energy to pair up electrons, so single electrons occupy different orbitals of the same energy (Hund’s Rule) ...
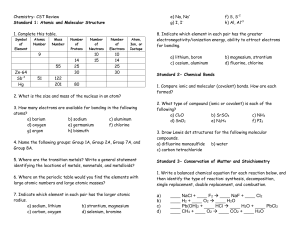
Atoms and Isotopes Worksheet
... 7. The # of protons in the nucleus of an atom is also known as the atomic _________ (mass/ number/ weight/ charge) 8. Which subatomic particles are found in the nucleus? ...
... 7. The # of protons in the nucleus of an atom is also known as the atomic _________ (mass/ number/ weight/ charge) 8. Which subatomic particles are found in the nucleus? ...
Single Replacement Reactions - Tri
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
Atomic Structure LO Teacher
... chemical nature of burning (combustion). He is also credited with providing the first experimental evidence for the law of conservation of mass, which states that… total mass of the products = total mass of the reactants In 1799, the French chemist Joseph Proust showed that the proportion by mass of ...
... chemical nature of burning (combustion). He is also credited with providing the first experimental evidence for the law of conservation of mass, which states that… total mass of the products = total mass of the reactants In 1799, the French chemist Joseph Proust showed that the proportion by mass of ...
Atomic Structure LO Teacher
... chemical nature of burning (combustion). He is also credited with providing the first experimental evidence for the law of conservation of mass, which states that… total mass of the products = total mass of the reactants In 1799, the French chemist Joseph Proust showed that the proportion by mass of ...
... chemical nature of burning (combustion). He is also credited with providing the first experimental evidence for the law of conservation of mass, which states that… total mass of the products = total mass of the reactants In 1799, the French chemist Joseph Proust showed that the proportion by mass of ...
Chapter 2 Atoms, Molecules, and Ions
... a. Dalton’s theory explained the laws of conservation of matter and constant composition. (How?) b. Dalton predicted the law of multiple proportions - two elements can form more than one compound by combining in different ratios. (This was later confirmed.) H2O and H2O2; CO and CO2 2.2 The Discovery ...
... a. Dalton’s theory explained the laws of conservation of matter and constant composition. (How?) b. Dalton predicted the law of multiple proportions - two elements can form more than one compound by combining in different ratios. (This was later confirmed.) H2O and H2O2; CO and CO2 2.2 The Discovery ...
Answer
... • Describe the periodic trends of either atomic radius or of ionisation energy. Explain the trend in the property selected. Atomic radius: Atomic radius decreases across a period and increases down a group. The numbers of protons and electrons increase as you move across a row. Electrons in s or p o ...
... • Describe the periodic trends of either atomic radius or of ionisation energy. Explain the trend in the property selected. Atomic radius: Atomic radius decreases across a period and increases down a group. The numbers of protons and electrons increase as you move across a row. Electrons in s or p o ...
Chapter 4 Atomic Structure
... a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have so little mass th ...
... a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have so little mass th ...
JEOPARDY
... levels? – *note* this works for science 1 class only because the third energy in reality contains more and you will learn this in chemistry class. Back to game board ...
... levels? – *note* this works for science 1 class only because the third energy in reality contains more and you will learn this in chemistry class. Back to game board ...
Chapter 5 - cloudfront.net
... corresponds to one exact frequency of light emitted by the atom Ground State – lowest possible energy of the electron in the Bohr model The light emitted by an electron moving from higher to a lower energy level has a frequency directly proportional to the energy change of the electron ...
... corresponds to one exact frequency of light emitted by the atom Ground State – lowest possible energy of the electron in the Bohr model The light emitted by an electron moving from higher to a lower energy level has a frequency directly proportional to the energy change of the electron ...
atomic structure jeopardy
... levels? – *note* this works for science 1 class only because the third energy in reality contains more and you will learn this in chemistry class. Back to game board ...
... levels? – *note* this works for science 1 class only because the third energy in reality contains more and you will learn this in chemistry class. Back to game board ...
Chemistry
... Chapter 4: Atomic Structure o Be able to identify elements on the periodic table o Know the basic structure of an atom o Be able to find the number of protons, electrons, neutrons, atomic number and mass number for a neutral atom, ion, and isotope o Know the diatomic gases ...
... Chapter 4: Atomic Structure o Be able to identify elements on the periodic table o Know the basic structure of an atom o Be able to find the number of protons, electrons, neutrons, atomic number and mass number for a neutral atom, ion, and isotope o Know the diatomic gases ...
Chapter 3 Atoms and the Periodic Table
... – electrons are going in the s orbital – Group 1 • H down to Fr • only one electron in the outer most energy level • 1 electron in the s orbital • these elements have 1 valence electron – Group 2 • Be down to Ra • 2 electrons in the outer most energy level • 2 electrons in the s orbital and it is no ...
... – electrons are going in the s orbital – Group 1 • H down to Fr • only one electron in the outer most energy level • 1 electron in the s orbital • these elements have 1 valence electron – Group 2 • Be down to Ra • 2 electrons in the outer most energy level • 2 electrons in the s orbital and it is no ...
1.1 Early Ideas of the Atom
... 2. According to Bohr’s theory, how can an electron gain or lose energy? 3. What happens when an electron in an excited atom returns to its ground state? 4. Bohr’s model of the atom is frequently referred to as the “quantum model.” Why? What does it mean to be quantized? How are electrons in atoms qu ...
... 2. According to Bohr’s theory, how can an electron gain or lose energy? 3. What happens when an electron in an excited atom returns to its ground state? 4. Bohr’s model of the atom is frequently referred to as the “quantum model.” Why? What does it mean to be quantized? How are electrons in atoms qu ...