Ionic Bond - hrsbstaff.ednet.ns.ca
... Atomic Mass • The atomic mass on the periodic table a weighted average of the isotopes ...
... Atomic Mass • The atomic mass on the periodic table a weighted average of the isotopes ...
chemical reaction
... Fe + O2 Fe2O3 Reaksiyonunda 11.2g Fe yeteri kadar oksijenle reaksiyona girdiğinde 10.0g Fe2O3 oluşuyor. Reaksiyonun teorik verimi, gerçek verimi ve yüzde verimini hesaplayınız. Fe :56 O:16 ...
... Fe + O2 Fe2O3 Reaksiyonunda 11.2g Fe yeteri kadar oksijenle reaksiyona girdiğinde 10.0g Fe2O3 oluşuyor. Reaksiyonun teorik verimi, gerçek verimi ve yüzde verimini hesaplayınız. Fe :56 O:16 ...
Chemistry Academic v. 2016
... Relate an element’s position on the periodic table to its electron configuration. Compare an element’s relativity to that of other elements. Describe chemical reactions in terms of atomic rearrangement and /or electron configuration. Explain how the periodicity of chemical properties led to the arra ...
... Relate an element’s position on the periodic table to its electron configuration. Compare an element’s relativity to that of other elements. Describe chemical reactions in terms of atomic rearrangement and /or electron configuration. Explain how the periodicity of chemical properties led to the arra ...
ppt
... Use the following thermochemical equation to calculate the molar heat of combustion of hexane. 2C6H14 (l) + 19O2 (g) 12CO2 (g) + 14H2O (g) + 7086kJ ...
... Use the following thermochemical equation to calculate the molar heat of combustion of hexane. 2C6H14 (l) + 19O2 (g) 12CO2 (g) + 14H2O (g) + 7086kJ ...
Chapter 7: Chemical Formulas and Chemical Compounds
... Chemical Names and Formulas A. There are millions of natural and synthetic compounds. Many times, the common names for them do not give any information on the chemical composition. To solve this problem, we give all compounds formulas that give this information. B. Significance of a Chemical Formula ...
... Chemical Names and Formulas A. There are millions of natural and synthetic compounds. Many times, the common names for them do not give any information on the chemical composition. To solve this problem, we give all compounds formulas that give this information. B. Significance of a Chemical Formula ...
+ H 2 (g)
... The ability of an element to react is referred to as the element’s activity. The more readily an element reacts with other substances, the greater its activity is. An activity series is a list of elements organized according to the ease with which the elements undergo single replacement reactions. ...
... The ability of an element to react is referred to as the element’s activity. The more readily an element reacts with other substances, the greater its activity is. An activity series is a list of elements organized according to the ease with which the elements undergo single replacement reactions. ...
Chemical Reactions
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
© NCERT not to be republished
... 38. When a brown compound of manganese (A) is treated with HCl it gives a gas (B). The gas taken in excess, reacts with NH3 to give an explosive compound (C). Identify compounds A, B and C. 39. Although fluorine is more electronegative than oxygen, but the ability of oxygen to stabilise higher oxida ...
... 38. When a brown compound of manganese (A) is treated with HCl it gives a gas (B). The gas taken in excess, reacts with NH3 to give an explosive compound (C). Identify compounds A, B and C. 39. Although fluorine is more electronegative than oxygen, but the ability of oxygen to stabilise higher oxida ...
Atomic Structure-1
... Some isotopes are more common than others. radioisotopes: unstable isotopes that emit radiation and decay into other isotopes ...
... Some isotopes are more common than others. radioisotopes: unstable isotopes that emit radiation and decay into other isotopes ...
In this experiment you will observe examples of the five basic types
... Ignite the alcohol from the top of the liquid with a Bunsen burner. Hold a cold watch glass well above the flame and observe the condensation of water on the bottom. The formation of the mist will be fleeting; watch closely. ...
... Ignite the alcohol from the top of the liquid with a Bunsen burner. Hold a cold watch glass well above the flame and observe the condensation of water on the bottom. The formation of the mist will be fleeting; watch closely. ...
2. Chapter 2
... is found combined with oxygen as water. Hydrogen is used in producing ammonia for fertilizers and for treating fossil fuels. Since it is lighter than air, hydrogen can be used to inflate weather balloons and to lift airships. Automobiles are now being made that can run on hydrogen gas instead of gas ...
... is found combined with oxygen as water. Hydrogen is used in producing ammonia for fertilizers and for treating fossil fuels. Since it is lighter than air, hydrogen can be used to inflate weather balloons and to lift airships. Automobiles are now being made that can run on hydrogen gas instead of gas ...
Subatomic Particles - Ciencias Esmeralda
... Thomson (1912) found 2 types of neon atoms and Soddy (1910) found 2 types of uranium atoms. 2 elements that have the same atomic number but different mass numbers Based on atomic structure: 2 elements that have the same number of protons but different number of neutrons. For example: Cl-35 and Cl-37 ...
... Thomson (1912) found 2 types of neon atoms and Soddy (1910) found 2 types of uranium atoms. 2 elements that have the same atomic number but different mass numbers Based on atomic structure: 2 elements that have the same number of protons but different number of neutrons. For example: Cl-35 and Cl-37 ...
Period:______ Table Number
... ________ (Uranium) are naturally occuring elements which can be found to exist somewhere in the earth’s land, water, or air. 57. On the periodic table of elements those elements which have an atomic number of ______ (Neptunium) to _____________ (Ununoctium) are synthetic or man made elements which h ...
... ________ (Uranium) are naturally occuring elements which can be found to exist somewhere in the earth’s land, water, or air. 57. On the periodic table of elements those elements which have an atomic number of ______ (Neptunium) to _____________ (Ununoctium) are synthetic or man made elements which h ...
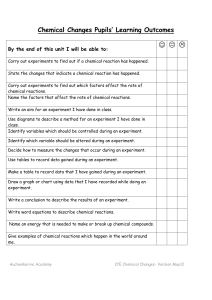
Chemical Reactions Unit Pupils` Learning Outcomes
... Identify variables which should be controlled during an experiment. Identify which variable should be altered during an experiment. Decide how to measure the changes that occur during an experiment. Use tables to record data gained during an experiment. Make a table to record data that I have gained ...
... Identify variables which should be controlled during an experiment. Identify which variable should be altered during an experiment. Decide how to measure the changes that occur during an experiment. Use tables to record data gained during an experiment. Make a table to record data that I have gained ...
ATOMIC STRUCTURE
... THAT RESIDE IN THE NUCLEUS AT THE CENTER OF THE ATOM • NEUTRONS ARE PARTICLES WITH NO CHARGE THAT ALSO ARE CONTAINED IN THE NUCLEUS. • ELECTRONS ARE NEGATIVELY CHARGED PARTICLES THAT ARE EXTERNAL TO THE NUCLEUS. ...
... THAT RESIDE IN THE NUCLEUS AT THE CENTER OF THE ATOM • NEUTRONS ARE PARTICLES WITH NO CHARGE THAT ALSO ARE CONTAINED IN THE NUCLEUS. • ELECTRONS ARE NEGATIVELY CHARGED PARTICLES THAT ARE EXTERNAL TO THE NUCLEUS. ...
Unit 6 – The Atom Vocabulary
... i) Electrons may _______________________ and temporarily move to a _____________ energy level. (1) This could be due to _____________________________________________ (2) This unstable state is called the ______________________________ ii) After the electron quickly returns to the ___________________ ...
... i) Electrons may _______________________ and temporarily move to a _____________ energy level. (1) This could be due to _____________________________________________ (2) This unstable state is called the ______________________________ ii) After the electron quickly returns to the ___________________ ...
Biochemistry unit notes part I
... of only one type of atom. – Cannot be chemically broken down to any other substances. – Are represented by chemical symbols on periodic table. – More than 100 elements are known, about 25 are found in living organisms. 6 most abundant include: O, C, H, N, P, S ...
... of only one type of atom. – Cannot be chemically broken down to any other substances. – Are represented by chemical symbols on periodic table. – More than 100 elements are known, about 25 are found in living organisms. 6 most abundant include: O, C, H, N, P, S ...
electron configuration
... furthest away from the nucleus. These are the valence electrons. • The valence electrons are the s and p electrons beyond the noble gas core. • For our purposes we will include ALL valence electrons past the noble gas core. ...
... furthest away from the nucleus. These are the valence electrons. • The valence electrons are the s and p electrons beyond the noble gas core. • For our purposes we will include ALL valence electrons past the noble gas core. ...
Atomic theory
... different element are different (Au atoms are different from Ag atoms) Atoms cannot be changed into different atoms by chemical of physical changes (Pb atoms cannot be turned into Au atoms) All atoms are like Billiard Balls – a solid, sphere Atoms react in whole numbers ...
... different element are different (Au atoms are different from Ag atoms) Atoms cannot be changed into different atoms by chemical of physical changes (Pb atoms cannot be turned into Au atoms) All atoms are like Billiard Balls – a solid, sphere Atoms react in whole numbers ...
2 KClO 3
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
AP Chapter 6 Powerpoint
... • Heisenberg’s uncertainty principle states that when an object has an extremely small mass (electron), it is impossible for us to simultaneously know both the exact momentum and location. • If the object is normal sized, then the uncertainty would be too small to matter. ...
... • Heisenberg’s uncertainty principle states that when an object has an extremely small mass (electron), it is impossible for us to simultaneously know both the exact momentum and location. • If the object is normal sized, then the uncertainty would be too small to matter. ...
Chemistry - Beachwood City Schools
... Answers to Chapter 11 Study Questions 1. Wavelength is inversely proportional to frequency. Energy is proportional to frequency. 2. The new idea in Bohr's model was that electrons can only exist in specific energy states. Bohr's model included an electron orbiting the nucleus as a planet does the su ...
... Answers to Chapter 11 Study Questions 1. Wavelength is inversely proportional to frequency. Energy is proportional to frequency. 2. The new idea in Bohr's model was that electrons can only exist in specific energy states. Bohr's model included an electron orbiting the nucleus as a planet does the su ...
CHEM%1212K% Final%Exam% Summer%2011% K
... C)%The%five%orbitals%remain%degenerate%but%have%a%higher%energy%than%before%% % ...
... C)%The%five%orbitals%remain%degenerate%but%have%a%higher%energy%than%before%% % ...