History of the Atom White Board Presentation
... English physicist, 1891-1974 Problem w/ previous model: mass of protons did not account for mass of nucleus and a nucleus of all protons (+) would repel and fall apart Experimental evidence: He bombarded metals (beryllium) with particles, causing a radiation that was both neutral and a mass sim ...
... English physicist, 1891-1974 Problem w/ previous model: mass of protons did not account for mass of nucleus and a nucleus of all protons (+) would repel and fall apart Experimental evidence: He bombarded metals (beryllium) with particles, causing a radiation that was both neutral and a mass sim ...
History of the Atom Notes Key
... English physicist, 1891-1974 Problem w/ previous model: mass of protons did not account for mass of nucleus and a nucleus of all protons (+) would repel and fall apart Experimental evidence: He bombarded metals (beryllium) with particles, causing a radiation that was both neutral and a mass sim ...
... English physicist, 1891-1974 Problem w/ previous model: mass of protons did not account for mass of nucleus and a nucleus of all protons (+) would repel and fall apart Experimental evidence: He bombarded metals (beryllium) with particles, causing a radiation that was both neutral and a mass sim ...
Fun With Predicting Reaction Products
... Predict the products of each of the following chemical reactions. If a reaction will not occur, explain why not. If a reaction does occur, balance it and state the type. Where a word equation is given, write the balanced chemical equation. If a chemical equation is given, write the names. ...
... Predict the products of each of the following chemical reactions. If a reaction will not occur, explain why not. If a reaction does occur, balance it and state the type. Where a word equation is given, write the balanced chemical equation. If a chemical equation is given, write the names. ...
Chapter 8 & 9 PowerPoint
... 1. Count valence electrons. 2. Connect atoms together with bonds. In molecules with a single atom of one element and several atoms of another element, the single atom is generally in the center with the other atoms attached to it. 3. Add electrons around outside of atoms to give each atom 8 electron ...
... 1. Count valence electrons. 2. Connect atoms together with bonds. In molecules with a single atom of one element and several atoms of another element, the single atom is generally in the center with the other atoms attached to it. 3. Add electrons around outside of atoms to give each atom 8 electron ...
review sheet
... o As you go down, you are increasing the number of energy levels on the atom, increasing its size. • Ionization energy (energy required to remove e- from atom in gas phase)…first ionization energy increases L to R, and decreases down the periodic table. o It gets harder to pull electrons from the at ...
... o As you go down, you are increasing the number of energy levels on the atom, increasing its size. • Ionization energy (energy required to remove e- from atom in gas phase)…first ionization energy increases L to R, and decreases down the periodic table. o It gets harder to pull electrons from the at ...
Unit 1: Sig. Figs, Compounds, Elements, Homo/Hetero mixtures
... 3. Carbon dioxide, water (H2O), and nitrous oxide are best characterized as a. atoms b. elements c. mixtures d. all chemicals e. molecules 4. Sand, air, and powdered iced tea are best characterized as a. atoms b. elements c. mixtures d. solutions e. molecules 5. The main difference between compounds ...
... 3. Carbon dioxide, water (H2O), and nitrous oxide are best characterized as a. atoms b. elements c. mixtures d. all chemicals e. molecules 4. Sand, air, and powdered iced tea are best characterized as a. atoms b. elements c. mixtures d. solutions e. molecules 5. The main difference between compounds ...
6.022 X 10 23 atoms - Fort Thomas Independent Schools
... chemical properties. Atoms of different elements have different physical and chemical properties. ...
... chemical properties. Atoms of different elements have different physical and chemical properties. ...
Chapter 7 Lecture
... into the surroundings it is a ??? reaction and has a + or – enthalpy? • The enthalpy of reaction for the combustion of CH4, the main component in natural gas: ...
... into the surroundings it is a ??? reaction and has a + or – enthalpy? • The enthalpy of reaction for the combustion of CH4, the main component in natural gas: ...
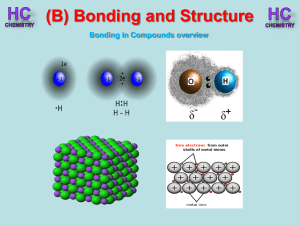
Lesson 1 - Bonding in compounds overview
... chemical formula is SiC. As this compound is linked by strong covalent bonding, it has a high m.p. (2700oC). It is a hard substance as it is very difficult to break the covalent lattice. SiC is used as an abrasive for smoothing very hard materials. Each Si is bonded to 4 C’s and each C is bonded to ...
... chemical formula is SiC. As this compound is linked by strong covalent bonding, it has a high m.p. (2700oC). It is a hard substance as it is very difficult to break the covalent lattice. SiC is used as an abrasive for smoothing very hard materials. Each Si is bonded to 4 C’s and each C is bonded to ...
Chapter 4 Notes
... these energies will not be radiated from the atom. Electronic Structure of Atoms ...
... these energies will not be radiated from the atom. Electronic Structure of Atoms ...
Bean Bag Lab
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
Class Slides
... light emitted from a low density cloud of gas. We call these features emission lines. Lines we see depend on compositions, density and ...
... light emitted from a low density cloud of gas. We call these features emission lines. Lines we see depend on compositions, density and ...
CHEMISTRY 1 CHAPTER II. ATOMIC STRUCTURE 2.1 ATOMIC
... Even though, it wasn´t very well accepted in his time, Democritus was the first to propose that atoms were the basic unit of all matter, it was the smallest indivisible part maintaining the characteristics of the original matter. Many years after John Dalton, took up this theory and support it it wi ...
... Even though, it wasn´t very well accepted in his time, Democritus was the first to propose that atoms were the basic unit of all matter, it was the smallest indivisible part maintaining the characteristics of the original matter. Many years after John Dalton, took up this theory and support it it wi ...
Mendeleev`s Periodic Table
... We have presented the contemporary quantum mechanical model of the atom. In using this model to describe the electronic structures of the elements in order of increasing atomic number, we saw that periodic similarities in electron configuration correlate with periodic similarities in properties, whi ...
... We have presented the contemporary quantum mechanical model of the atom. In using this model to describe the electronic structures of the elements in order of increasing atomic number, we saw that periodic similarities in electron configuration correlate with periodic similarities in properties, whi ...
Precipitation Reactions
... Acid-Base Reactions: Thinking synthetically •Acid-base neutralization reactions are good methods to create solutions of soluble salts. •The cation of the salt is the metal in the metal hydroxide (base). •The anion of the salt is the anion of the acid. •The driving force of this reaction is so stron ...
... Acid-Base Reactions: Thinking synthetically •Acid-base neutralization reactions are good methods to create solutions of soluble salts. •The cation of the salt is the metal in the metal hydroxide (base). •The anion of the salt is the anion of the acid. •The driving force of this reaction is so stron ...
Review - cloudfront.net
... Which of the following has the greatest heat capacity? a. 1000 g of water c. 1 g of water b. 1000 g of steel d. 1 g of steel The amount of heat transferred from an object depends on which of the following? a. the specific heat of the object c. the mass of the object b. the initial temperature of the ...
... Which of the following has the greatest heat capacity? a. 1000 g of water c. 1 g of water b. 1000 g of steel d. 1 g of steel The amount of heat transferred from an object depends on which of the following? a. the specific heat of the object c. the mass of the object b. the initial temperature of the ...
1 Brief History of Medical Imaging
... Between 0.5 and 10-2 °A, (°A = 10-10 m), corresponding to photon energies of about 25 KeV to 1:0 MeV, the attenuation is at reasonable levels with a wavelength far shorter than the resolution of interest. This is desirable since it ensures that diffraction will not in any way distort the imaging sys ...
... Between 0.5 and 10-2 °A, (°A = 10-10 m), corresponding to photon energies of about 25 KeV to 1:0 MeV, the attenuation is at reasonable levels with a wavelength far shorter than the resolution of interest. This is desirable since it ensures that diffraction will not in any way distort the imaging sys ...
Atoms and Molecules
... HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole ratios needed] ...
... HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole ratios needed] ...
Chapter 6 - Atomic Theory
... Frequency = speed of light / wavelength – Different frequencies of light have different energy levels –E = hν (Energy = Planck’s constant x frequency) • High frequency light has high energy (violet light) • Low frequency light has low energy (red light) ...
... Frequency = speed of light / wavelength – Different frequencies of light have different energy levels –E = hν (Energy = Planck’s constant x frequency) • High frequency light has high energy (violet light) • Low frequency light has low energy (red light) ...
Document
... If the colliding electrons have an energy between that of level 2 and level 3 when they hit the atom then… A. No light will come from the atom B. One color of light will be emitted from the atom C. Two colors of light will be emitted from the atom D. Three colors of light will be emitted from the at ...
... If the colliding electrons have an energy between that of level 2 and level 3 when they hit the atom then… A. No light will come from the atom B. One color of light will be emitted from the atom C. Two colors of light will be emitted from the atom D. Three colors of light will be emitted from the at ...
Atoms and Elements Practice Test Chemistry
... E) none of the above 15) Isotopes are: A) atoms of the same element that have the same number of neutrons. B) atoms of the same element that have different number of neutrons. C) atoms of the same element that have different number of electrons. D) atoms of the same element that have different numbe ...
... E) none of the above 15) Isotopes are: A) atoms of the same element that have the same number of neutrons. B) atoms of the same element that have different number of neutrons. C) atoms of the same element that have different number of electrons. D) atoms of the same element that have different numbe ...
Unit 10 – The Mole
... Unit 10 – The Mole Calculating Formula, Atomic, and Molecular Masses (MOLAR MASS) As you know, the mass of an element on the periodic table is a weighted average of all the naturallyoccurring isotopes of that element. Originally, we said that the unit for the mass of an element was ____________. Fro ...
... Unit 10 – The Mole Calculating Formula, Atomic, and Molecular Masses (MOLAR MASS) As you know, the mass of an element on the periodic table is a weighted average of all the naturallyoccurring isotopes of that element. Originally, we said that the unit for the mass of an element was ____________. Fro ...
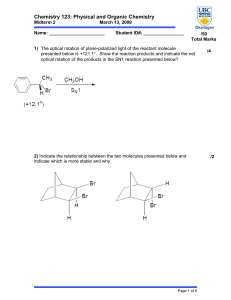
Chemistry 123: Physical and Organic Chemistry
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...