Nature of chemical reaction - Environmental-Chemistry
... Energy and chemical reactions: • Chemical reactions are breaking of old bonds from reactant-molecules and formation of new bonds in product-molecules. • Chemical reactions involve changes in energy. Photosynthesis is an endothermic reaction. • Energy is released (exothermic) during formation of bon ...
... Energy and chemical reactions: • Chemical reactions are breaking of old bonds from reactant-molecules and formation of new bonds in product-molecules. • Chemical reactions involve changes in energy. Photosynthesis is an endothermic reaction. • Energy is released (exothermic) during formation of bon ...
Chapter 5 CHEM 121
... numerous forms such as the electrical energy released by the chemical reactions of an ordinary cell phone battery. Often, all or most of the energy takes the form of heat. • Chemical reactions that release heat as a product are called exothermic reactions. Ordinary combustion of a log in a fireplace ...
... numerous forms such as the electrical energy released by the chemical reactions of an ordinary cell phone battery. Often, all or most of the energy takes the form of heat. • Chemical reactions that release heat as a product are called exothermic reactions. Ordinary combustion of a log in a fireplace ...
Measuring of the cosmic ray
... This relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent, while those that radiate weakly endure longer. Half-lives of known radionuclides vary widely, from more than 1019 years (such as for very nearly stable nuclides, e.g. 209Bi), to ...
... This relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent, while those that radiate weakly endure longer. Half-lives of known radionuclides vary widely, from more than 1019 years (such as for very nearly stable nuclides, e.g. 209Bi), to ...
Name - TeacherWeb
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
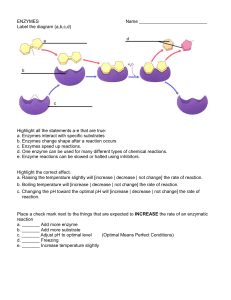
ENZYMES
... Highlight all the statements a-e that are true: a. Enzymes interact with specific substrates b. Enzymes change shape after a reaction occurs c. Enzymes speed up reactions. d. One enzyme can be used for many different types of chemical reactions. e. Enzyme reactions can be slowed or halted using inhi ...
... Highlight all the statements a-e that are true: a. Enzymes interact with specific substrates b. Enzymes change shape after a reaction occurs c. Enzymes speed up reactions. d. One enzyme can be used for many different types of chemical reactions. e. Enzyme reactions can be slowed or halted using inhi ...
1 - PTO
... 3. Using the arrow notations to represent guests, diagram what the hotel would look like with 34 guests. You can diagram this on the first hotel blueprint. Make sure to follow all of the rules! 4. Using the arrow notations to represent guests, diagram what the hotel would look like with 45 guests. Y ...
... 3. Using the arrow notations to represent guests, diagram what the hotel would look like with 34 guests. You can diagram this on the first hotel blueprint. Make sure to follow all of the rules! 4. Using the arrow notations to represent guests, diagram what the hotel would look like with 45 guests. Y ...
atoms - Wappingers Central School
... most a particles went straight through without hitting anything, BUT a few were deflected, this means a particles must have hit something: ...
... most a particles went straight through without hitting anything, BUT a few were deflected, this means a particles must have hit something: ...
Worksheet 1 - Oxidation/Reduction Reactions Oxidation number
... Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example. Hydrob ...
... Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example. Hydrob ...
AP Chemistry Summer Assignment Summer 2015 Ms. Osquist
... • Determine if an element is a metal or nonmetal and the charge of its most common ion (for A-group elements) using the periodic table. • Write the formula of an ionic compound given its name (including polyatomic ions). • Explain how a cation or anion would form from a neutral atom. • Identify the ...
... • Determine if an element is a metal or nonmetal and the charge of its most common ion (for A-group elements) using the periodic table. • Write the formula of an ionic compound given its name (including polyatomic ions). • Explain how a cation or anion would form from a neutral atom. • Identify the ...
atom
... • The Nucleus Protons are positively charged particles in the nucleus. Neutrons are the particles of the nucleus that have no electrical charge. • Outside the Nucleus Electrons are the negatively charged particles in atoms. Electrons are found around the nucleus within electron clouds. All the struc ...
... • The Nucleus Protons are positively charged particles in the nucleus. Neutrons are the particles of the nucleus that have no electrical charge. • Outside the Nucleus Electrons are the negatively charged particles in atoms. Electrons are found around the nucleus within electron clouds. All the struc ...
Balancing a Chemical Equation
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
Elements, Mixtures and Compounds
... A group of non-metal atoms held together by covalent bonds is called a molecule. Molecules made up of only two atoms are called diatomic molecules, e.g. hydrogen chloride. HCl (one carbon atom and one chlorine atom), and carbon monoxide, CO, (one carbon atom and one oxygen atom). Certain elements no ...
... A group of non-metal atoms held together by covalent bonds is called a molecule. Molecules made up of only two atoms are called diatomic molecules, e.g. hydrogen chloride. HCl (one carbon atom and one chlorine atom), and carbon monoxide, CO, (one carbon atom and one oxygen atom). Certain elements no ...
Balancing a Chemical Equation
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
Balancing a Chemical Equation
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
Atoms: The Building Blocks of Matter
... proportion, and the law of multiple proportions. • He reasoned that elements are composed of one kind of atom and that only whole numbers of two or more kinds of atoms can combine to form compounds. • He proposed the solid sphere model ...
... proportion, and the law of multiple proportions. • He reasoned that elements are composed of one kind of atom and that only whole numbers of two or more kinds of atoms can combine to form compounds. • He proposed the solid sphere model ...
Chemistry - Bourbon County Schools
... Use the periodic table to determine the atomic number; atomic mass; mass number; and number of protons, electrons, and neutrons in isotopes of elements Calculate the weighted average atomic mass of an element from isotopic abundance, given the atomic mass of each contributor Identify regions (e.g., ...
... Use the periodic table to determine the atomic number; atomic mass; mass number; and number of protons, electrons, and neutrons in isotopes of elements Calculate the weighted average atomic mass of an element from isotopic abundance, given the atomic mass of each contributor Identify regions (e.g., ...
balancing eqns teacher
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
... Some helpful hints for balancing equations: Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, doub ...
AQA Additional Sci C2 Revision Guide
... outer shell of its atoms. Elements in groups 1 and 2 of the periodic table only have 1 or 2 electrons in their outer shells so these form positive ions by losing their outer electrons. Elements in groups 6 and 7 of the periodic table only need 1 or 2 electrons to fill up their outer shells so these ...
... outer shell of its atoms. Elements in groups 1 and 2 of the periodic table only have 1 or 2 electrons in their outer shells so these form positive ions by losing their outer electrons. Elements in groups 6 and 7 of the periodic table only need 1 or 2 electrons to fill up their outer shells so these ...
Archived Lecture Notes #1 - Atomic and Electronic Structure
... Consider the atom of an element containing one extra-nuclear electron: hydrogen; for electro-neutrality the charge on the nucleus must be +1. This orbiting electron (in the ground state - the lowest possible, most stable state) will have the lowest available quantum numbers in n, l and m. That is, n ...
... Consider the atom of an element containing one extra-nuclear electron: hydrogen; for electro-neutrality the charge on the nucleus must be +1. This orbiting electron (in the ground state - the lowest possible, most stable state) will have the lowest available quantum numbers in n, l and m. That is, n ...
chemistry-2nd-edition-julia-burdge-solution
... When writing formulas of ionic compounds, the subscript of the cation is numerically equal to the charge of the anion, and the subscript of the anion is numerically equal to the charge on the cation. If the charges of the cation and anion are numerically equal, then no subscripts are necessary. Char ...
... When writing formulas of ionic compounds, the subscript of the cation is numerically equal to the charge of the anion, and the subscript of the anion is numerically equal to the charge on the cation. If the charges of the cation and anion are numerically equal, then no subscripts are necessary. Char ...
Ch. 5 PPT Part 1
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and ...
AP CHEMISTRY SUMMER ASSIGNMENT
... An atom is composed of a nucleus which contains the protons and neutrons. Although the nucleus makes up nearly all of the mass of the atom, it accounts for only 1/10,000th of the size of the atom. ...
... An atom is composed of a nucleus which contains the protons and neutrons. Although the nucleus makes up nearly all of the mass of the atom, it accounts for only 1/10,000th of the size of the atom. ...