t2 – modern atomic theory continued
... space in which negatively charged electrons are suspended in a positive spongy matrix. The results of Rutherford’s experiments, however, were very different. Most of the α-particles passed through the gold foil unaffected while a significant number of them deflected off course at large angles with a ...
... space in which negatively charged electrons are suspended in a positive spongy matrix. The results of Rutherford’s experiments, however, were very different. Most of the α-particles passed through the gold foil unaffected while a significant number of them deflected off course at large angles with a ...
Quantum Mechanics PPT
... • As protons are added one by one to the nucleus to build up the elements, electrons are similarly added to orbitals • Electrons fill in low energy orbitals before high energy orbitals ...
... • As protons are added one by one to the nucleus to build up the elements, electrons are similarly added to orbitals • Electrons fill in low energy orbitals before high energy orbitals ...
Final Exam Practice
... a. electrons. b. atoms and molecules. c. found only in the plasma state. d. as of yet undiscovered. ____ 69. Protons and neutrons are found grouped together in the: a. electron cloud. b. charge. c. periodic table. d. nucleus. ____ 70. Protons and neutrons in the nucleus are held together by: a. grav ...
... a. electrons. b. atoms and molecules. c. found only in the plasma state. d. as of yet undiscovered. ____ 69. Protons and neutrons are found grouped together in the: a. electron cloud. b. charge. c. periodic table. d. nucleus. ____ 70. Protons and neutrons in the nucleus are held together by: a. grav ...
Types of Chemical Reactions
... Divide the smallest number of moles of an element into the moles of each element present. Convert the fractional ratios for each element into whole numbers by multiplying all the ratios by the same number. The resulting numbers are the subscripts for the each element in the empirical formula. ...
... Divide the smallest number of moles of an element into the moles of each element present. Convert the fractional ratios for each element into whole numbers by multiplying all the ratios by the same number. The resulting numbers are the subscripts for the each element in the empirical formula. ...
Atomic Theory and the Nuclear Atom
... A. In approximately 400 B.C.E., the idea that all matter is made up of extremely small, solid, _____________________ particles called atoms was first proposed by Greek thinkers, such as _________________. B. In 1808 C.E., John ______________ expanded on this theory and proposed an atomic theory with ...
... A. In approximately 400 B.C.E., the idea that all matter is made up of extremely small, solid, _____________________ particles called atoms was first proposed by Greek thinkers, such as _________________. B. In 1808 C.E., John ______________ expanded on this theory and proposed an atomic theory with ...
Chapter 4 Early Atomic Theory
... If two elements form more that one compound, the ratio of the second element that combines with 1 gram of the first element in each is a simple whole number. • In hydrogen peroxide 32.0 g oxygen reacts with 2.0 g hydrogen (H2O2) O:H = 16:1 • Ratio of the masses of oxygen in hydrogen peroxide and wat ...
... If two elements form more that one compound, the ratio of the second element that combines with 1 gram of the first element in each is a simple whole number. • In hydrogen peroxide 32.0 g oxygen reacts with 2.0 g hydrogen (H2O2) O:H = 16:1 • Ratio of the masses of oxygen in hydrogen peroxide and wat ...
Curriculum Plan
... relationships and conversions Understand and agree to lab safety rules; identify and know how to use safety equipment Understand: Definitions, the Laws of Conservation of Energy and of Conservation of Matter, Temperature scales and conversions, Chemical and physical changes, Classifying Matter, Chem ...
... relationships and conversions Understand and agree to lab safety rules; identify and know how to use safety equipment Understand: Definitions, the Laws of Conservation of Energy and of Conservation of Matter, Temperature scales and conversions, Chemical and physical changes, Classifying Matter, Chem ...
Mass/Mole Conversions
... • Isotopes: atoms of the same element that have different masses due to different numbers of neutrons. • Mass Number: the total number of protons and neutrons that make up the nucleus of an isotope ~ Isotopes are written with the mass number written after the element name or symbol with a hyphen: ex ...
... • Isotopes: atoms of the same element that have different masses due to different numbers of neutrons. • Mass Number: the total number of protons and neutrons that make up the nucleus of an isotope ~ Isotopes are written with the mass number written after the element name or symbol with a hyphen: ex ...
Silicon vs. Carbon - Coristines
... *Electronegativity describes the ability of an atom to attract electrons towards itself in a covalent bond. An atom's electronegativity is affected by both its atomic weight and the distance that its valence electrons are from the nucleus. The higher the electronegativity number, the more an element ...
... *Electronegativity describes the ability of an atom to attract electrons towards itself in a covalent bond. An atom's electronegativity is affected by both its atomic weight and the distance that its valence electrons are from the nucleus. The higher the electronegativity number, the more an element ...
chemical reaction
... Balancing Chemical Equations • When you write the chemical equation for a reaction, you must observe the law of conservation of mass. • When you count the number of carbon, hydrogen, oxygen, and sodium atoms on each side of the arrow in the equation, you find equal numbers of each kind of atom. ...
... Balancing Chemical Equations • When you write the chemical equation for a reaction, you must observe the law of conservation of mass. • When you count the number of carbon, hydrogen, oxygen, and sodium atoms on each side of the arrow in the equation, you find equal numbers of each kind of atom. ...
Classifying Chemical Reactions by What Atoms Do
... For reactions that are not metal + nonmetal, or do not involve O2, we need a method for determining how the electrons are transferred. Chemists assign a number to each element in a reaction called an oxidation state that allows them to determine the electron flow in the reaction. Even though they lo ...
... For reactions that are not metal + nonmetal, or do not involve O2, we need a method for determining how the electrons are transferred. Chemists assign a number to each element in a reaction called an oxidation state that allows them to determine the electron flow in the reaction. Even though they lo ...
Chapter 7: Atomic Structure and the Periodic Table
... explains why of the two electron configurations shown below for nitrogen (N), only the one on the right is correct. ...
... explains why of the two electron configurations shown below for nitrogen (N), only the one on the right is correct. ...
1411_lecture_ch2
... Rutherford. He noticed that when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He t ...
... Rutherford. He noticed that when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He t ...
Chapter 20: Electrochemistry
... -Assign each element an Oxidation Number (State). If any element changes it’s oxidation state during a reaction, it is a redox reaction Zero Oxidation States indicate element is neutral [+] Oxidation States indicate element is “electron poor” [-] Oxidation States indicate element is “electron rich” ...
... -Assign each element an Oxidation Number (State). If any element changes it’s oxidation state during a reaction, it is a redox reaction Zero Oxidation States indicate element is neutral [+] Oxidation States indicate element is “electron poor” [-] Oxidation States indicate element is “electron rich” ...
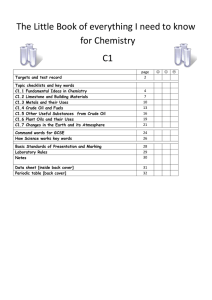
The Big book of C1 chemistry
... Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon, for example iron oxide is reduced in the blast furnace to make iron. [reduction is removal of oxygen] Metals that are more reactive than carbon, such as aluminium, are extracted by electrolysis of ...
... Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon, for example iron oxide is reduced in the blast furnace to make iron. [reduction is removal of oxygen] Metals that are more reactive than carbon, such as aluminium, are extracted by electrolysis of ...
DOC
... fields alter each other's courses slightly, are regarded as occupying separate orbits. Shell number 2 can have a total of eight electrons which are divided into two subshells. Two a electrons form a spherical configuration again; but, unlike the first shell pair, these 2s electrons combine chemicall ...
... fields alter each other's courses slightly, are regarded as occupying separate orbits. Shell number 2 can have a total of eight electrons which are divided into two subshells. Two a electrons form a spherical configuration again; but, unlike the first shell pair, these 2s electrons combine chemicall ...
Bonding - IGChemistry
... Some bonds are not purely ionic, but they still have a significant difference in electronegativity that leads to one atom pulling the electrons more strongly than the other. ...
... Some bonds are not purely ionic, but they still have a significant difference in electronegativity that leads to one atom pulling the electrons more strongly than the other. ...
Atomic Theory - Northwest ISD Moodle
... 1. The cathode rays had the same properties regardless of the metal used for the cathode. 2. The rays traveled from the cathode (- charged) to the anode (+ charged). 3. The rays were attracted to the positive plate of an external electrical field, and repelled by the ...
... 1. The cathode rays had the same properties regardless of the metal used for the cathode. 2. The rays traveled from the cathode (- charged) to the anode (+ charged). 3. The rays were attracted to the positive plate of an external electrical field, and repelled by the ...
r - Purdue Physics
... •For a given number of protons there is a nucleus that is most stable for a particular number of neutrons. •Isotopes are when for the same number of protons the number of neutrons is different from the most stable configuration. •Since the number of electrons is the same the chemical properties are ...
... •For a given number of protons there is a nucleus that is most stable for a particular number of neutrons. •Isotopes are when for the same number of protons the number of neutrons is different from the most stable configuration. •Since the number of electrons is the same the chemical properties are ...
Calculations with Chemical Formulas and Equations
... • The limiting reactant is the reactant present in the smallest stoichiometric amount – In other words, it’s the reactant you’ll run out of first (in this case, the H2) ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount – In other words, it’s the reactant you’ll run out of first (in this case, the H2) ...
Atomic structure
... no matter how much or how little of the compound you have. These proportions are in _____________________; for example every water molecule has two hydrogen atoms for each oxygen atom (H2O). You would not say that there is 1 hydrogen for each 1/2 oxygen. So when elements combine during chemical reac ...
... no matter how much or how little of the compound you have. These proportions are in _____________________; for example every water molecule has two hydrogen atoms for each oxygen atom (H2O). You would not say that there is 1 hydrogen for each 1/2 oxygen. So when elements combine during chemical reac ...