1 2016-17 Honors Chemistry Review for the Final Exam Each unit
... g) The molecule is known to be 518 times more soluble in carbon tetrachloride than water. Does this support or conflict with the assumption about the shape of the molecule from “f” above? Explain your statement by clearly discussing the involved chemicals. What does this indicate about the type of ...
... g) The molecule is known to be 518 times more soluble in carbon tetrachloride than water. Does this support or conflict with the assumption about the shape of the molecule from “f” above? Explain your statement by clearly discussing the involved chemicals. What does this indicate about the type of ...
Structure of the Atom Today`s DCI
... The number located below the element’s symbol. No units listed in the periodic table…we must remember the units of the atomic mass are atomic mass units. The symbol for atomic mass units is u. For hydrogen the atomic mass is 1.00797 u. The mass of a hydrogen atom is 1.6736 x 10-24 g. ...
... The number located below the element’s symbol. No units listed in the periodic table…we must remember the units of the atomic mass are atomic mass units. The symbol for atomic mass units is u. For hydrogen the atomic mass is 1.00797 u. The mass of a hydrogen atom is 1.6736 x 10-24 g. ...
WEEK
... atoms and the formation of ions Students will be able to identify nuclear reactions as nuclear decay, nuclear fission, or nuclear fusion. Students will be able to describe the relative amounts of energy produced in nuclear ...
... atoms and the formation of ions Students will be able to identify nuclear reactions as nuclear decay, nuclear fission, or nuclear fusion. Students will be able to describe the relative amounts of energy produced in nuclear ...
Final Review
... 4. What is the volume of one mole of gas at STP? a. 1 liter b. 12 liters c. 22.4 liters d. It depends on the gas since all gases have different densities 5. Which of the following is NOT true at STP conditions? a. Temperature is at 0°C and pressure is at 1 atm b. Temperature is at 273 K and pressure ...
... 4. What is the volume of one mole of gas at STP? a. 1 liter b. 12 liters c. 22.4 liters d. It depends on the gas since all gases have different densities 5. Which of the following is NOT true at STP conditions? a. Temperature is at 0°C and pressure is at 1 atm b. Temperature is at 273 K and pressure ...
Atoms and electrons
... for properties such as atomic radius, first ionization energy, and electron affinity (from previous studies) ...
... for properties such as atomic radius, first ionization energy, and electron affinity (from previous studies) ...
Chapter 4- Elements and the Periodic Table
... formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means "uncuttable;' for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The ancient Greeks did not prove the existence of atoms because they did not do exp ...
... formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means "uncuttable;' for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The ancient Greeks did not prove the existence of atoms because they did not do exp ...
Writing Chemical Formulas and Chemical Reactions
... A binary acid is a binary chemical compound containing hydrogen and a nonmetal from Group 6 or 7. These compounds can be named using the regular naming system for binary molecular compounds if they are gases. But, binary acids are usually found as clear, viscous liquids at room temperature and a dif ...
... A binary acid is a binary chemical compound containing hydrogen and a nonmetal from Group 6 or 7. These compounds can be named using the regular naming system for binary molecular compounds if they are gases. But, binary acids are usually found as clear, viscous liquids at room temperature and a dif ...
Elements and the Periodic Table
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
Atomic Structure
... Discovery of the Atomic Nucleus • More detail of the atom’s structure was provided in 1911 by Ernest Rutherford and his associates Hans Geiger and Ernest Marsden. • The results of their gold foil experiment led to the discovery of a very densely packed bundle of matter with a positive electric charg ...
... Discovery of the Atomic Nucleus • More detail of the atom’s structure was provided in 1911 by Ernest Rutherford and his associates Hans Geiger and Ernest Marsden. • The results of their gold foil experiment led to the discovery of a very densely packed bundle of matter with a positive electric charg ...
Webquest: Atomic Theories and Models
... My famous quote was disputed by Aristotle, although time proved me correct. In what date and who proposed atomic theory. Name the date and inventor who developed laws of electrolysis. Arranged elements into 7 groups When and who developed an electrical device to click when hit with alpha particles. ...
... My famous quote was disputed by Aristotle, although time proved me correct. In what date and who proposed atomic theory. Name the date and inventor who developed laws of electrolysis. Arranged elements into 7 groups When and who developed an electrical device to click when hit with alpha particles. ...
Atomic Theory Webquest
... Your team has been chosen to defend one of the significant scientists who contributed to the current ideas surrounding atomic theory. While on CNN, the International Foundation of Scientific Theory accused these men to be pseudoscientists stating that their work, while it lead to the current theory ...
... Your team has been chosen to defend one of the significant scientists who contributed to the current ideas surrounding atomic theory. While on CNN, the International Foundation of Scientific Theory accused these men to be pseudoscientists stating that their work, while it lead to the current theory ...
Chapter 3- sec 2- Structure of the atom
... Discovery of the Atomic Nucleus • More detail of the atom’s structure was provided in 1911 by Ernest Rutherford and his associates Hans Geiger and Ernest Marsden. • The results of their gold foil experiment led to the discovery of a very densely packed bundle of matter with a positive electric charg ...
... Discovery of the Atomic Nucleus • More detail of the atom’s structure was provided in 1911 by Ernest Rutherford and his associates Hans Geiger and Ernest Marsden. • The results of their gold foil experiment led to the discovery of a very densely packed bundle of matter with a positive electric charg ...
Atomic Theory WebQuest PDF
... Your team has been chosen to defend one of the significant scientists who contributed to the current ideas surrounding atomic theory. While on CNN, the International Foundation of Scientific Theory accused these men to be pseudoscientists stating that their work, while it lead to the current theory ...
... Your team has been chosen to defend one of the significant scientists who contributed to the current ideas surrounding atomic theory. While on CNN, the International Foundation of Scientific Theory accused these men to be pseudoscientists stating that their work, while it lead to the current theory ...
CHEMICAL REACTIONS
... 3. reactions with acids : a. carbonates or bicarbonates and acids form a salt, water and CO2 • e.g. 2HCl + Na2CO3 Æ 2 NaCl + H2O + CO2 (net : H+ + CO32- Æ H2O + CO2) b. sulfites and acids form a salt, water and SO2 • e.g. 2 HCl + Na2SO3 Æ 2 NaCl + H2O + SO2 (net : H+ + SO32- Æ H2O + SO2) c. metallic ...
... 3. reactions with acids : a. carbonates or bicarbonates and acids form a salt, water and CO2 • e.g. 2HCl + Na2CO3 Æ 2 NaCl + H2O + CO2 (net : H+ + CO32- Æ H2O + CO2) b. sulfites and acids form a salt, water and SO2 • e.g. 2 HCl + Na2SO3 Æ 2 NaCl + H2O + SO2 (net : H+ + SO32- Æ H2O + SO2) c. metallic ...
Project Advance Chemistry 106 Sample Questions
... when 0.84 moles of PCl5 is placed in a 3.0 L flask, it was found that when the reaction reaches equilibrium, 0.72 moles of PCl5 still remains. What is the value of the equilibrium constant for this reaction? A. B. C. D. E. ...
... when 0.84 moles of PCl5 is placed in a 3.0 L flask, it was found that when the reaction reaches equilibrium, 0.72 moles of PCl5 still remains. What is the value of the equilibrium constant for this reaction? A. B. C. D. E. ...
Atomic Structure and Function
... • Gas (no definite shape nor definite volume) • Liquid (definite volume but no definite shape) • Solid (definite shape and definite volume) ...
... • Gas (no definite shape nor definite volume) • Liquid (definite volume but no definite shape) • Solid (definite shape and definite volume) ...
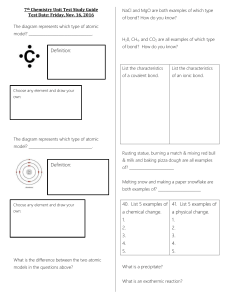
7th Chemistry Unit Test Study Guide Test Date: Friday, Nov. 16
... _____________________ are the substances that you end up with in a chemical reaction and are found to the right of the arrow. _____________________ are the substances that you begin with in a chemical reaction and are found to the left of the arrow. What does the arrow mean in a chemical reaction? ...
... _____________________ are the substances that you end up with in a chemical reaction and are found to the right of the arrow. _____________________ are the substances that you begin with in a chemical reaction and are found to the left of the arrow. What does the arrow mean in a chemical reaction? ...
"ALICE" CHAPTER 12 MODERN VIEW OF ATOMIC STRUCTURE
... {13}______________. As a result, {14}________________ of an element are atoms that have different masses. The atomic masses that appear on the periodic table are merely "weighted averages" of the masses of all the naturally-occurring isotopes. Weighted averages are determined by including the relati ...
... {13}______________. As a result, {14}________________ of an element are atoms that have different masses. The atomic masses that appear on the periodic table are merely "weighted averages" of the masses of all the naturally-occurring isotopes. Weighted averages are determined by including the relati ...
Redox Balancing Worksheet
... The oxidation number of any pure element is zero. Thus the oxidation number of H in H2 is zero. The oxidation number of a monatomic ion is equal to its charge. Thus the oxidation number of Cl in the Clion is -1, that for Mg in the Mg+2 ion is +2, and that for oxygen in O2- ion is -2. The sum of the ...
... The oxidation number of any pure element is zero. Thus the oxidation number of H in H2 is zero. The oxidation number of a monatomic ion is equal to its charge. Thus the oxidation number of Cl in the Clion is -1, that for Mg in the Mg+2 ion is +2, and that for oxygen in O2- ion is -2. The sum of the ...
Chemistry basics powerpoint Chapter 2
... Identify the four major elements 2. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass number, atomic weight and mass number 3. Distinguish between and discuss the biological importance of the following: nonpolar covalent ...
... Identify the four major elements 2. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass number, atomic weight and mass number 3. Distinguish between and discuss the biological importance of the following: nonpolar covalent ...
2013 atoms
... Parts of the Atom Because of Dalton’s atomic theory, most scientists in the 1800s believed that the atom was like a tiny solid ball that could not be broken up into parts. In 1897, a British physicist, J.J. Thomson, discovered that this solidball model was not accurate. ...
... Parts of the Atom Because of Dalton’s atomic theory, most scientists in the 1800s believed that the atom was like a tiny solid ball that could not be broken up into parts. In 1897, a British physicist, J.J. Thomson, discovered that this solidball model was not accurate. ...