Structure and Properties of Matter Jeopardy
... Based on the periodic table, which of the following elements has physical and chemical properties most like 25% ...
... Based on the periodic table, which of the following elements has physical and chemical properties most like 25% ...
Chemical Reactions
... • Balanced chemical equations – same number of atoms of each element appear on each side of the equation – stoichiometric coefficients - needed to balance the equations ...
... • Balanced chemical equations – same number of atoms of each element appear on each side of the equation – stoichiometric coefficients - needed to balance the equations ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
APES Ch. 3 Notes
... b. Low Quality Matter – disorganized, dilute, deep underground or dispersed in oceans or the atmosphere, and has little potential for use as a matter resource. Entropy – measure of the disorder or randomness of a system or its environment ...
... b. Low Quality Matter – disorganized, dilute, deep underground or dispersed in oceans or the atmosphere, and has little potential for use as a matter resource. Entropy – measure of the disorder or randomness of a system or its environment ...
State Standard - SchoolNotes.com
... compounds by using Lewis dot structures and oxidation numbers. C-3.2 Interpret the names and formulas for ionic and covalent compounds. C-3.3 Explain how the types of intermolecular forces present in a compound affect the physical properties of compounds (including polarity and molecular shape). C-3 ...
... compounds by using Lewis dot structures and oxidation numbers. C-3.2 Interpret the names and formulas for ionic and covalent compounds. C-3.3 Explain how the types of intermolecular forces present in a compound affect the physical properties of compounds (including polarity and molecular shape). C-3 ...
FYBSc Revised Syllabus
... 2.5.2. Acetylation of amines with acetic anhydride and acetyl chloride, Action of nitrous acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respec ...
... 2.5.2. Acetylation of amines with acetic anhydride and acetyl chloride, Action of nitrous acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respec ...
Balancing Redox Reactions 1 - VCC Library
... electrons lost in the oxidation process must equal the total number of electrons gained during the reduction process. In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substanc ...
... electrons lost in the oxidation process must equal the total number of electrons gained during the reduction process. In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substanc ...
File
... 46. Using the equation you balanced in the problem above, determine how many moles of calcium carbide are needed to react completely with 49.0 grams of H2O. 47. The last step in the production of nitric acid is the reaction of nitrogen dioxide with water. 3NO2 (g) + H2O (l) 2HNO3 (aq) + NO (g) How ...
... 46. Using the equation you balanced in the problem above, determine how many moles of calcium carbide are needed to react completely with 49.0 grams of H2O. 47. The last step in the production of nitric acid is the reaction of nitrogen dioxide with water. 3NO2 (g) + H2O (l) 2HNO3 (aq) + NO (g) How ...
Living Things - Peoria Public Schools
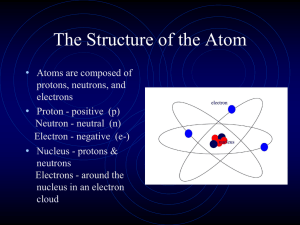
... particles called neutrons. • In the current atomic theory, electrons do not move in circular paths around the nucleus as Bohr thought. • Instead, the current theory suggests that electrons move within an area around the nucleus called the electron cloud. ...
... particles called neutrons. • In the current atomic theory, electrons do not move in circular paths around the nucleus as Bohr thought. • Instead, the current theory suggests that electrons move within an area around the nucleus called the electron cloud. ...
File - StarpointLearns
... 13. Explain, in terms of subatomic particles, why an oxide ion, O2-, has a negative charge. 14. Compare the number of protons to the number of electrons in a positive ion. 15. Compare the number of protons to the number of electrons in a negative ion. 16. Explain, in terms of subatomic particles, wh ...
... 13. Explain, in terms of subatomic particles, why an oxide ion, O2-, has a negative charge. 14. Compare the number of protons to the number of electrons in a positive ion. 15. Compare the number of protons to the number of electrons in a negative ion. 16. Explain, in terms of subatomic particles, wh ...
Name: 1) What is the oxidation number of sulfur in H SO ? A)
... 33) In the reaction MnO2 + 4HCl ‡‡ˆ MnCl 2 + 2H2O + Cl 2, which species is reduced? A) O2- ...
... 33) In the reaction MnO2 + 4HCl ‡‡ˆ MnCl 2 + 2H2O + Cl 2, which species is reduced? A) O2- ...
General Chemistry I - University of Toledo
... 5.6 Compare the wavelength and frequency of different electron transitions in the Bohr model of the atom. 5.7 Relate wavelengths calculated using the Balmer-Rydberg equation to energy levels in the Bohr model of the atom. 5.8 Calculate the wavelength of a moving object using the de Broglie equation. ...
... 5.6 Compare the wavelength and frequency of different electron transitions in the Bohr model of the atom. 5.7 Relate wavelengths calculated using the Balmer-Rydberg equation to energy levels in the Bohr model of the atom. 5.8 Calculate the wavelength of a moving object using the de Broglie equation. ...
water, h2o
... The s orbitals are of course spherically symmetric, while the p orbitals are of the form: px = sinθ∙cosφ; py = sinθ∙sinφ; pz = cosθ Since another 2 electrons from 2 hydrogens will fill the 2p shell you would guess that H2O would be a happy molecule and quite inert. However, simple valence counting d ...
... The s orbitals are of course spherically symmetric, while the p orbitals are of the form: px = sinθ∙cosφ; py = sinθ∙sinφ; pz = cosθ Since another 2 electrons from 2 hydrogens will fill the 2p shell you would guess that H2O would be a happy molecule and quite inert. However, simple valence counting d ...
Notes - The Models of the Atom
... James Chadwick, discoverer of the neutron V. Rutherford’s model + Neutrons A. The nucleus contains most of the atom's ______________ as well as the _____________ charge. B. At the time it was believed that protons supposedly accounted for this _______________. C. However, a nucleus with twice the ch ...
... James Chadwick, discoverer of the neutron V. Rutherford’s model + Neutrons A. The nucleus contains most of the atom's ______________ as well as the _____________ charge. B. At the time it was believed that protons supposedly accounted for this _______________. C. However, a nucleus with twice the ch ...
Section 1 Powerpoint
... • He predicted that most particles would travel in a straight path from their source to a screen that lit up when struck. ...
... • He predicted that most particles would travel in a straight path from their source to a screen that lit up when struck. ...
Unit 2.2 Test Review Key
... evidences of a chemical reaction is shown. Use what you know to logically work each of these problems!! Examples of state of matter changes (These are NOT chemical reactions!) Water – liquid to solid to water vapor (steam) Aluminum – we can shape it into a soda can, melt it, freeze it, but it is ...
... evidences of a chemical reaction is shown. Use what you know to logically work each of these problems!! Examples of state of matter changes (These are NOT chemical reactions!) Water – liquid to solid to water vapor (steam) Aluminum – we can shape it into a soda can, melt it, freeze it, but it is ...
Thermodynamics Test Study Guide—AP _____ 1. The entropy
... heat of water is 1.00 cal/g-oC and that no heat is lost to or gained from the surroundings, what is the specific heat of copper, in cal/g-oC? 11. The combustion of 0.100 gram of ethane causes a temperature rise of 2.00oC in a bomb calorimeter that has a heat capacity of 2.510 kJ/oC. What is the inte ...
... heat of water is 1.00 cal/g-oC and that no heat is lost to or gained from the surroundings, what is the specific heat of copper, in cal/g-oC? 11. The combustion of 0.100 gram of ethane causes a temperature rise of 2.00oC in a bomb calorimeter that has a heat capacity of 2.510 kJ/oC. What is the inte ...
CHEM 1405 Practice Exam #2
... 19) Which of the statements below best describes the following reaction? Na2CO3(s) Na2O(s) + CO2(g) A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxi ...
... 19) Which of the statements below best describes the following reaction? Na2CO3(s) Na2O(s) + CO2(g) A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxi ...
Document
... Three moles of 1-propanol, C3H7OH, reacts with one mole of phosphorus trichloride to produce 1-chloropropane, C3H7Cl, and phosphorus acid, H3PO3. What is the percent yield if you begin with 75.0 g of both 1propanol and phosphorus trichloride and obtain 1.0 mole of 1-chloropropane? (1propanol= 60.10 ...
... Three moles of 1-propanol, C3H7OH, reacts with one mole of phosphorus trichloride to produce 1-chloropropane, C3H7Cl, and phosphorus acid, H3PO3. What is the percent yield if you begin with 75.0 g of both 1propanol and phosphorus trichloride and obtain 1.0 mole of 1-chloropropane? (1propanol= 60.10 ...
Chapter 1 Chemistry: The Study of Matter
... more different kinds of atoms. The atoms are held together by bonds to form molecules. Molecules can be broken down by chemical methods. When compounds are broken down, the components have completely different properties than the compound. ...
... more different kinds of atoms. The atoms are held together by bonds to form molecules. Molecules can be broken down by chemical methods. When compounds are broken down, the components have completely different properties than the compound. ...
half-reactions - Clayton State University
... - Plating of iron with chromium - Anodic protection (metal is oxidized under controlled conditions) - Cathodic protection (a more reactive metal is placed in contact) ...
... - Plating of iron with chromium - Anodic protection (metal is oxidized under controlled conditions) - Cathodic protection (a more reactive metal is placed in contact) ...
Isotopes
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
Electric Charge - sdeleonadvancedphysics
... over one body making it positively charged, while the other kind of fluid spreads over the other body, making it negative charged. ...
... over one body making it positively charged, while the other kind of fluid spreads over the other body, making it negative charged. ...
Document
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...