Notes section 5.2
... Hydrogen Atomic Orbitals • Just as Bohr’s model had numbers assigned to electron orbits, so does the quantum mechanical model. There are 4 quantum numbers for orbitals. • Principal quantum number (n) indicates the relative size and energy of atomic orbitals. • n specifies the atom’s major energy le ...
... Hydrogen Atomic Orbitals • Just as Bohr’s model had numbers assigned to electron orbits, so does the quantum mechanical model. There are 4 quantum numbers for orbitals. • Principal quantum number (n) indicates the relative size and energy of atomic orbitals. • n specifies the atom’s major energy le ...
Introduction to particle physics
... in two or more ways forming substances C and D, then if mass A is held constant, the masses of B in the various products will be related in proportions that are the ratios of small integers” Conclude: when elementary substances combine, they do so as discrete entities or atoms Dalton’s atomic theory ...
... in two or more ways forming substances C and D, then if mass A is held constant, the masses of B in the various products will be related in proportions that are the ratios of small integers” Conclude: when elementary substances combine, they do so as discrete entities or atoms Dalton’s atomic theory ...
Measuring Energy Changes In A Chemical Reaction Sept. 2016
... If we assume that: heat lost/gained by the system = heat gained/lost by the surroundings then we can experimentally determine the energy changes in chemical reactions ...
... If we assume that: heat lost/gained by the system = heat gained/lost by the surroundings then we can experimentally determine the energy changes in chemical reactions ...
COUNTING ATOMS
... number of each atom present. B. A representation of a chemical reaction expressed as a formula. C. Substances that change in a reaction. D. The new substances that are formed as a result of the reaction. ...
... number of each atom present. B. A representation of a chemical reaction expressed as a formula. C. Substances that change in a reaction. D. The new substances that are formed as a result of the reaction. ...
king fahd university of petroleum and minerals chemistry
... 35. Which statement is NOT correct about transition metals? A) B) C) D) ...
... 35. Which statement is NOT correct about transition metals? A) B) C) D) ...
EXAM 3
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
5 - Particles in an atom
... Substances that contain only one kind of atom are called elements. Some familiar elements are oxygen, gold, silver, and helium. An atom is the smallest part of an element that can be broken down and still have the characteristics of that element. All atoms are basically the same. All atoms of the sa ...
... Substances that contain only one kind of atom are called elements. Some familiar elements are oxygen, gold, silver, and helium. An atom is the smallest part of an element that can be broken down and still have the characteristics of that element. All atoms are basically the same. All atoms of the sa ...
No Slide Title - McMaster Chemistry
... Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
... Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
Lecture 6
... Stability of a phase (or mineral) is partly related to its internal energy (here “E”), which strives to be as low as possible under the external conditions. Metastability exists in a phase when its energy is higher than P-T conditions indicate it should be. (1) Activation Energy is the energy ...
... Stability of a phase (or mineral) is partly related to its internal energy (here “E”), which strives to be as low as possible under the external conditions. Metastability exists in a phase when its energy is higher than P-T conditions indicate it should be. (1) Activation Energy is the energy ...
TDDFT as a tool in chemistry and biochemistry
... […] Photochemistry may also be introduced to laymen as a reaction that proceeds with the absorption of light. Normally a reaction (not just a photochemical reaction) occurs when a molecule gains the necessary activation energy to undergo change. A simple example can be the combustion of gasoline (a ...
... […] Photochemistry may also be introduced to laymen as a reaction that proceeds with the absorption of light. Normally a reaction (not just a photochemical reaction) occurs when a molecule gains the necessary activation energy to undergo change. A simple example can be the combustion of gasoline (a ...
Stuff Matters Handout
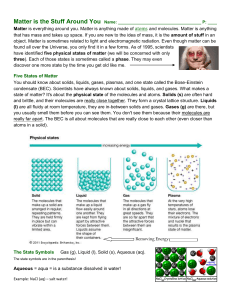
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
g - Porterville College Home
... otherwise, fill in the fields and register a new account. 4. Find your course in the list (you may need to expand the subject and term categories) and click the link. 5. Use the bookstore package to register for the online homework or select a payment option and follow the remaining instructions. On ...
... otherwise, fill in the fields and register a new account. 4. Find your course in the list (you may need to expand the subject and term categories) and click the link. 5. Use the bookstore package to register for the online homework or select a payment option and follow the remaining instructions. On ...
Chapter3 Solutions
... ∆EN = EN P − EN Ca = 2.19 − 1.00 = 1.19 , covalent 4. This statement is true, because in general, the farther away elements are from one another, the greater is the difference in their electronegativity, and the more likely they are to form ionic bonds. Students should note that noble gases are an e ...
... ∆EN = EN P − EN Ca = 2.19 − 1.00 = 1.19 , covalent 4. This statement is true, because in general, the farther away elements are from one another, the greater is the difference in their electronegativity, and the more likely they are to form ionic bonds. Students should note that noble gases are an e ...
9th class bridge course 74-112
... energy and fall inside the nucleus. But nucleus is found to be stable. Thus Rutherford’s atomic model does not explain the stability of an atom. ...
... energy and fall inside the nucleus. But nucleus is found to be stable. Thus Rutherford’s atomic model does not explain the stability of an atom. ...
File
... • Unreactive elements have the exact number of electrons needed to fill their outer energy level. ...
... • Unreactive elements have the exact number of electrons needed to fill their outer energy level. ...
4.2 Discovering Parts of the Atom
... • Unreactive elements have the exact number of electrons needed to fill their outer energy level. ...
... • Unreactive elements have the exact number of electrons needed to fill their outer energy level. ...
Ch. 6: Chemical Reactions Study Guide
... In endothermic reactions energy is transferred from the surroundings into the reactants. An endothermic reaction is one in which heat is transferred from the surroundings to the reactants. In an exothermic reaction, energy is transferred from the reactants to the surroundings. A chemical reaction th ...
... In endothermic reactions energy is transferred from the surroundings into the reactants. An endothermic reaction is one in which heat is transferred from the surroundings to the reactants. In an exothermic reaction, energy is transferred from the reactants to the surroundings. A chemical reaction th ...
Unit 2 - The Atom 1-3.key
... this information, Which isotope on of hydrogen must be the mostwhich abundant, based on this data? ____ isotope must be the most abundant? ...
... this information, Which isotope on of hydrogen must be the mostwhich abundant, based on this data? ____ isotope must be the most abundant? ...
CHEMISTRY 1
... The Born- Haber cycle uses the law of Hess to determine the Lattice Energy. The lattice energy is the enthalphy change, ∆H, associated when gaseous cations and anions from a crystal: Na+(g) + Cl-(g) NaCl(s) ∆H = - 788KJ Since heat is always evolved in these processes, all lattice energies have a n ...
... The Born- Haber cycle uses the law of Hess to determine the Lattice Energy. The lattice energy is the enthalphy change, ∆H, associated when gaseous cations and anions from a crystal: Na+(g) + Cl-(g) NaCl(s) ∆H = - 788KJ Since heat is always evolved in these processes, all lattice energies have a n ...