Ch. 14 notes (teacher)3
... gases – ___________ are not listed in Table 14.2 since form _____________ compounds ! they do not ________ ...
... gases – ___________ are not listed in Table 14.2 since form _____________ compounds ! they do not ________ ...
400 Chem periodic table
... • Is the net positive charge experienced by electron in multielectron atom. • Protons always added to nucleus, but electron positions can change as atomic number increases (energy levels). • Three factors control the net charge: size of energy levels, nuclear charge and the shielding effect. • These ...
... • Is the net positive charge experienced by electron in multielectron atom. • Protons always added to nucleus, but electron positions can change as atomic number increases (energy levels). • Three factors control the net charge: size of energy levels, nuclear charge and the shielding effect. • These ...
Homework Answers - Chemistry from AZ
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
Chapter 6:
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...
Chapter 3: Atoms and the periodic table
... group of atoms. Some energy levels are only partially filled, which makes it easier for them to gain or lose electrons in order to have a full outer energy level. If an atom gains or loses an electron, it no longer has the same number of electrons as it does protons. Because the charges do not cance ...
... group of atoms. Some energy levels are only partially filled, which makes it easier for them to gain or lose electrons in order to have a full outer energy level. If an atom gains or loses an electron, it no longer has the same number of electrons as it does protons. Because the charges do not cance ...
ANSWERS-ATOMIC STRUCTURE WORKSHEET
... An electron arrangement is how many electrons are around the nucleus and where there are. Electron arrangement definitely does affect the chemical properties of an atom. For example, if we take a look at Group 18, the noble gases, they all have a full valance shell. It doesn’t matter whether you mov ...
... An electron arrangement is how many electrons are around the nucleus and where there are. Electron arrangement definitely does affect the chemical properties of an atom. For example, if we take a look at Group 18, the noble gases, they all have a full valance shell. It doesn’t matter whether you mov ...
Lectures 14-15 - U of L Class Index
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
Lecture 14-15 - U of L Class Index
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
Test Review
... a noble gas. Too reactive to exist in nature freely. Must be stored in mineral oil. Alkaline Earth Metals – Group 2, not as reactive as alkali metals. Form +2 ions, losing 2 electrons to look like a noble gas. Halogens – Group 7, most reactive metals on the periodic table. Form -1 ions, gaining 1 el ...
... a noble gas. Too reactive to exist in nature freely. Must be stored in mineral oil. Alkaline Earth Metals – Group 2, not as reactive as alkali metals. Form +2 ions, losing 2 electrons to look like a noble gas. Halogens – Group 7, most reactive metals on the periodic table. Form -1 ions, gaining 1 el ...
Word - The Chemistry Book
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
Jeopardy Game
... in a chemical compound to attract electrons from another atom in the compound is called this. ...
... in a chemical compound to attract electrons from another atom in the compound is called this. ...
Periodic Trends
... Many properties of the elements tend to change in a predictable way, known as trends. Trends among elements in the periodic table include their sizes and their abilities to lose or attract electrons. Atomic Size Definition: Atomic size is related the atomic radius of an element, which is defined as ...
... Many properties of the elements tend to change in a predictable way, known as trends. Trends among elements in the periodic table include their sizes and their abilities to lose or attract electrons. Atomic Size Definition: Atomic size is related the atomic radius of an element, which is defined as ...
File
... 2. Identify the family on the periodic table in which each member has a full valence energy level. 3. Identify the alkaline earth metal with valence electrons in the 3rd energy level. 4. Identify a family on the periodic table with 7 valence electrons. 5. Write an abbreviated electron configuration ...
... 2. Identify the family on the periodic table in which each member has a full valence energy level. 3. Identify the alkaline earth metal with valence electrons in the 3rd energy level. 4. Identify a family on the periodic table with 7 valence electrons. 5. Write an abbreviated electron configuration ...
Chapter 6 notes
... There are _____________ rows, or periods, in the table. Each period corresponds to a principal energy level. The elements within a ___________________, or group, in the periodic table have similar properties. The pattern of properties within a period repeats as you move from one period to the next. ...
... There are _____________ rows, or periods, in the table. Each period corresponds to a principal energy level. The elements within a ___________________, or group, in the periodic table have similar properties. The pattern of properties within a period repeats as you move from one period to the next. ...
Chapter 4: The Periodic Table
... elements? How do each of these trends progress as you move across a period? Atomic size increases, ionization energy decreases, and electron affinity decreases from top to bottom in a group. Atomic size decreases, ionization energy increases, and electron affinity increases from left to right across ...
... elements? How do each of these trends progress as you move across a period? Atomic size increases, ionization energy decreases, and electron affinity decreases from top to bottom in a group. Atomic size decreases, ionization energy increases, and electron affinity increases from left to right across ...
Document
... I can create graphs using the Excel software. I can analyze trends across the periods and down the groups by interpreting the graphs. ...
... I can create graphs using the Excel software. I can analyze trends across the periods and down the groups by interpreting the graphs. ...
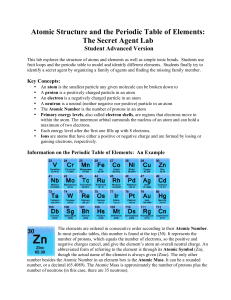
Atomic Structure and the Periodic Table of Elements: The Secret
... 4. Once you have several distinct piles focusing on one or two shared characteristics, now try looking at the big picture and combine the agents into one large family portrait. 5. If you have compiled the Secret Agents into the correct arrangement, there will be one empty spot in the family portrait ...
... 4. Once you have several distinct piles focusing on one or two shared characteristics, now try looking at the big picture and combine the agents into one large family portrait. 5. If you have compiled the Secret Agents into the correct arrangement, there will be one empty spot in the family portrait ...
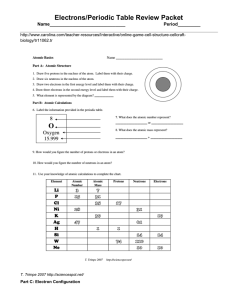
Electrons/Periodic Table Review Packet Name______________________________ Period_________
... Electrons/Periodic Table Review Packet Name______________________________ b. metals with unpredictable properties c. a halogen ...
... Electrons/Periodic Table Review Packet Name______________________________ b. metals with unpredictable properties c. a halogen ...
Periodic Table – Organizing the Elements
... & are poor conductors of heat and electricity Nonmetals can be solid (C & S), liquid (Br) or gas (O & H) ...
... & are poor conductors of heat and electricity Nonmetals can be solid (C & S), liquid (Br) or gas (O & H) ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
Standards Practice
... number and atomic mass. Use the periodic table on page 56 to answer questions 1-5. 1. The atomic number in the periodic table is highest in the A. upper left comer. B. upper right comer. C. lower left comer. D. lower right comer. 2. The atomic mass in the periodic table is highest in the A. upper le ...
... number and atomic mass. Use the periodic table on page 56 to answer questions 1-5. 1. The atomic number in the periodic table is highest in the A. upper left comer. B. upper right comer. C. lower left comer. D. lower right comer. 2. The atomic mass in the periodic table is highest in the A. upper le ...
Adv Chem Multiple Choice Practice: The Periodic Table
... ____ 32. Which of the following elements has the smallest ionic radius? a. Li b. K c. O d. S ____ 33. What is the energy required to remove an electron from an atom called? a. nuclear energy b. ionization energy c. shielding energy d. electronegative energy ____ 34. Which of the following factors co ...
... ____ 32. Which of the following elements has the smallest ionic radius? a. Li b. K c. O d. S ____ 33. What is the energy required to remove an electron from an atom called? a. nuclear energy b. ionization energy c. shielding energy d. electronegative energy ____ 34. Which of the following factors co ...
Topic 3-Periodicity
... Obtain evidence for scientific theories by making and testing predictions based on them—scientists organize subjects based on structure and function; the periodic table is a key example of this. Early models of the periodic table from Mendeleev, and later Moseley, allowed for the prediction of prope ...
... Obtain evidence for scientific theories by making and testing predictions based on them—scientists organize subjects based on structure and function; the periodic table is a key example of this. Early models of the periodic table from Mendeleev, and later Moseley, allowed for the prediction of prope ...