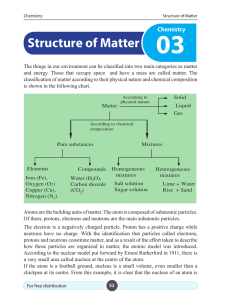
Structure of Matter - e
... The atomic number is the number of protons in an atom of the element. Atomic number of the element = number of protons in an atom of the element For example, there are 11 protons in the nucleus of a sodium atom. Thus, the atomic number of sodium is 11.The number of protons in every atom of the same ...
... The atomic number is the number of protons in an atom of the element. Atomic number of the element = number of protons in an atom of the element For example, there are 11 protons in the nucleus of a sodium atom. Thus, the atomic number of sodium is 11.The number of protons in every atom of the same ...
KD 1
... their discussion report to the other groups while the other groups compare the information with the information they get by gallery walk Have them to walk around the class and see other group display and giving comment related to the topic Review the displays and comments Give information of t ...
... their discussion report to the other groups while the other groups compare the information with the information they get by gallery walk Have them to walk around the class and see other group display and giving comment related to the topic Review the displays and comments Give information of t ...
Ch 6 Notes
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...
unit iv – the periodic table
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
File - Chemical Engineering
... Other alternative periodic tables exist:Some versions of the table show a dark stair-step line along the metalloids. Metals are to the left of the line and non-metals to the right. The layout of the periodic table demonstrates recurring ("periodic") chemical properties. Elements are listed in order ...
... Other alternative periodic tables exist:Some versions of the table show a dark stair-step line along the metalloids. Metals are to the left of the line and non-metals to the right. The layout of the periodic table demonstrates recurring ("periodic") chemical properties. Elements are listed in order ...
Alkali metals
... and used pattern to predict properties of undiscovered elements such as eka-aluminum (Gallium) and eka-silicon (Germanium). ...
... and used pattern to predict properties of undiscovered elements such as eka-aluminum (Gallium) and eka-silicon (Germanium). ...
Document
... stronger than the alkali metals. They also have higher melting points. They are less reactive than alkali metals, but they too are too reactive to be found free in nature. ...
... stronger than the alkali metals. They also have higher melting points. They are less reactive than alkali metals, but they too are too reactive to be found free in nature. ...
Periodic Table - Doral Academy Preparatory
... ionization energy, electronegativity, and state the reasons for these variations. 2. What are valence electrons, and how many are present in atoms of each main-group element? 3. Compare the atomic radii, ionization energies, and electronegativities of the d-block elements with those of the main-grou ...
... ionization energy, electronegativity, and state the reasons for these variations. 2. What are valence electrons, and how many are present in atoms of each main-group element? 3. Compare the atomic radii, ionization energies, and electronegativities of the d-block elements with those of the main-grou ...
Algebra - Militant Grammarian
... elements, shown as dots, and each colored different colors, shrink as the groups near the noble gases. In other words, the noble gases are the smallest dots. This is from left to right. If the picture is looked at from top to bottom, the dots grow larger as they near the bottom. The radii of the ele ...
... elements, shown as dots, and each colored different colors, shrink as the groups near the noble gases. In other words, the noble gases are the smallest dots. This is from left to right. If the picture is looked at from top to bottom, the dots grow larger as they near the bottom. The radii of the ele ...
Periodic Table – Organizing the Elements
... electricity, most are solids Nonmetals do not have luster & are poor conductors ...
... electricity, most are solids Nonmetals do not have luster & are poor conductors ...
Periodic Table - Doral Academy Preparatory
... ionization energy, electronegativity, and state the reasons for these variations. 2. What are valence electrons, and how many are present in atoms of each main-group element? 3. Compare the atomic radii, ionization energies, and electronegativities of the d-block elements with those of the main-grou ...
... ionization energy, electronegativity, and state the reasons for these variations. 2. What are valence electrons, and how many are present in atoms of each main-group element? 3. Compare the atomic radii, ionization energies, and electronegativities of the d-block elements with those of the main-grou ...
Chapter Three: Periodic Table
... The Periodic Law states that the properties of the elements are a period function of their atomic number. This means that, when the elements are arranged by atomic number, those with similar properties will be at regular intervals; these elements will be in the same group. ...
... The Periodic Law states that the properties of the elements are a period function of their atomic number. This means that, when the elements are arranged by atomic number, those with similar properties will be at regular intervals; these elements will be in the same group. ...
Periodic Properties of Elements
... Elements which ionize (lose electrons) readily are metals: Sodium, Iron, Lead Elements which readily gain electrons are non-metals: Chlorine, Sulphur, Argon Separating them are the metalloids: Boron, Silicon, Arsenic ...
... Elements which ionize (lose electrons) readily are metals: Sodium, Iron, Lead Elements which readily gain electrons are non-metals: Chlorine, Sulphur, Argon Separating them are the metalloids: Boron, Silicon, Arsenic ...
Chapter_3_Fast_Facts
... Atomic radius: atomic radii decrease along a period as the nuclear charge increases and electrons are added to the same outer shell. The attraction between the outer electrons and nucleus increases. ...
... Atomic radius: atomic radii decrease along a period as the nuclear charge increases and electrons are added to the same outer shell. The attraction between the outer electrons and nucleus increases. ...
Periodic trends
... VERY reactive because one valence e• Found as compounds in nature • Not including H! ...
... VERY reactive because one valence e• Found as compounds in nature • Not including H! ...
The Periodic Table
... • These elements have very low melting and boiling temperatures and all are gases at room temperature. • Their lack of reactivity is due to the electron configuration. Each noble gas has an outer shell that is considered ‘full’ or stable. • As such they do not want to react with other elements and u ...
... • These elements have very low melting and boiling temperatures and all are gases at room temperature. • Their lack of reactivity is due to the electron configuration. Each noble gas has an outer shell that is considered ‘full’ or stable. • As such they do not want to react with other elements and u ...
Chapter 8 Reading Guide Name: AP Chemistry 2016
... 37. Explain how to determine the number of electrons in an anion. Use O2- as an example. 38. Explain how to determine the number of electrons in a cation. Use Na+ as an example. ...
... 37. Explain how to determine the number of electrons in an anion. Use O2- as an example. 38. Explain how to determine the number of electrons in a cation. Use Na+ as an example. ...
Unit 4 Notes
... • They have typical metallic properties such as conduction of electricity and high luster. • Less reactive than group 1 and 2 elements. • Some (i.e. platinum & gold) are so unreactive they usually don’t form compounds. ...
... • They have typical metallic properties such as conduction of electricity and high luster. • Less reactive than group 1 and 2 elements. • Some (i.e. platinum & gold) are so unreactive they usually don’t form compounds. ...
TEST-Periodic Table
... b. mixture of aluminum and an unknown element. c. unknown element he predicted would have properties similar to those of aluminum. d. rare isotope of aluminum. ...
... b. mixture of aluminum and an unknown element. c. unknown element he predicted would have properties similar to those of aluminum. d. rare isotope of aluminum. ...
Periodic Table Trends - Magoffin County Schools
... • As the number of PROTONS and ELECTRONS INCREASES, the attraction between them pulls them closer together, making the atoms SMALLER. ...
... • As the number of PROTONS and ELECTRONS INCREASES, the attraction between them pulls them closer together, making the atoms SMALLER. ...
17.3 The periodic table
... The periodic law The periodic law states that when the elements are arranged by increasing the atomic number, a periodic repetition of chemical and physical properties of the elements are noticed. ...
... The periodic law The periodic law states that when the elements are arranged by increasing the atomic number, a periodic repetition of chemical and physical properties of the elements are noticed. ...
Homework Packet - Chemistry from AZ
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
Periodic Trends Note Packet Key
... Valence Electrons: Electrons that are able to be gained, lost, or shared in the formation of chemical compounds. The s and p electrons in the outermost energy level. ...
... Valence Electrons: Electrons that are able to be gained, lost, or shared in the formation of chemical compounds. The s and p electrons in the outermost energy level. ...
4-2 A Guided Tour of the Periodic Table
... An amu is equal to one-twelfth of the mass of a carbon-12 atom. This isotope of carbon has six protons and six neutrons, so individual protons and neutrons must each have a mass of about 1.0 amu, because electrons contribute very little mass. ...
... An amu is equal to one-twelfth of the mass of a carbon-12 atom. This isotope of carbon has six protons and six neutrons, so individual protons and neutrons must each have a mass of about 1.0 amu, because electrons contribute very little mass. ...
Chapter 6
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...
... regular pattern, or a periodic pattern. Mendeleev placed the known elements in a table, where he arranged elements into columns with similar properties. ...