Chapter 6 the periodic table
... Most nonmetals are gases at room temperature. Some are solids and one is a liquid Hard to generalize because there are many different properties Opposite of metal – Poor conductors – Solid nonmetals tend to be brittle. ...
... Most nonmetals are gases at room temperature. Some are solids and one is a liquid Hard to generalize because there are many different properties Opposite of metal – Poor conductors – Solid nonmetals tend to be brittle. ...
The Periodic Table - Palisades High School
... periodic table (cont) • Dimitri Mendeleev, in 1869, used this information and produced the first orderly arrangement of all 63 known elements. • He arranged them in a similar way to Newlands and created the first periodic table. • Mendeleev started a new row each time he noticed that the chemical pr ...
... periodic table (cont) • Dimitri Mendeleev, in 1869, used this information and produced the first orderly arrangement of all 63 known elements. • He arranged them in a similar way to Newlands and created the first periodic table. • Mendeleev started a new row each time he noticed that the chemical pr ...
Jeopardy
... a. the number of protons b. the number of neutrons c. the number of electrons in the innermost energy level d. the number of electrons in the outermost energy level ...
... a. the number of protons b. the number of neutrons c. the number of electrons in the innermost energy level d. the number of electrons in the outermost energy level ...
Atomic Structure and the Periodic Table of Elements: The Secret
... o The group number—usually depicted by a Roman numeral above the group—refers to the number of valence electrons in the outermost electron shell. Ex: Group IA elements (Alkali Metals) have one valence electron in their outermost shells. o The period number refers to the number of electron shells or ...
... o The group number—usually depicted by a Roman numeral above the group—refers to the number of valence electrons in the outermost electron shell. Ex: Group IA elements (Alkali Metals) have one valence electron in their outermost shells. o The period number refers to the number of electron shells or ...
Chemistry - • Elements • Electron Configurations • The Periodic Table
... can be calculated from quantum theory in a very complex way. The results can be summarized very simply: Elements would like to have 8 electrons in their outermost energy level (highest n value). This means that elements try to get a s2p6 configuration in their highest n value energy level. This is t ...
... can be calculated from quantum theory in a very complex way. The results can be summarized very simply: Elements would like to have 8 electrons in their outermost energy level (highest n value). This means that elements try to get a s2p6 configuration in their highest n value energy level. This is t ...
Regions of the Periodic Table
... (The “extra” section below the rest of the table.) are part of the transition metals have a partially-filled f sub-level officially have 2 valence electrons, but can shift electrons between s, d, and f sub-levels. Usually form ions with +3 charges. are rare noble gases: elements in group 18 ...
... (The “extra” section below the rest of the table.) are part of the transition metals have a partially-filled f sub-level officially have 2 valence electrons, but can shift electrons between s, d, and f sub-levels. Usually form ions with +3 charges. are rare noble gases: elements in group 18 ...
b. - s3.amazonaws.com
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
Year 9 study the new AQA GCSE specification for first examination
... The elements in Group 0 of the periodic table are called the noble gases. They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons. The noble gases have eight electrons in their outer energy level, except for helium, which has only two electrons. ...
... The elements in Group 0 of the periodic table are called the noble gases. They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons. The noble gases have eight electrons in their outer energy level, except for helium, which has only two electrons. ...
What makes a group of elements
... The alkali metals are so soft that they can be cut with a knife. The freshly cut surface of an alkali metal is shiny, but it dulls quickly as the metal reacts with oxygen and water in the air. ...
... The alkali metals are so soft that they can be cut with a knife. The freshly cut surface of an alkali metal is shiny, but it dulls quickly as the metal reacts with oxygen and water in the air. ...
to Ch 05 Periodic Trends
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
1. Which of the following is the most important - Hatboro
... 8. The _____________________ have a single electron in the highest energy level? 9. The _____________________ achieve the electron configurations of noble gases by losing two electrons. 10. The _____________________ vary in the number of electrons in the highest energy level. 11. The _______________ ...
... 8. The _____________________ have a single electron in the highest energy level? 9. The _____________________ achieve the electron configurations of noble gases by losing two electrons. 10. The _____________________ vary in the number of electrons in the highest energy level. 11. The _______________ ...
Atoms and periodic properties
... single capitalized letter for their symbol • The rest, that have permanent names have two letters. – the first is capitalized and the second is lower case – the lower case letter is either the second letter in the name, or the letter of a strong consonant heard when the name of the element is spoken ...
... single capitalized letter for their symbol • The rest, that have permanent names have two letters. – the first is capitalized and the second is lower case – the lower case letter is either the second letter in the name, or the letter of a strong consonant heard when the name of the element is spoken ...
Document
... The atomic mass of each atom represents an average of all of the individual isotopes of that element. Two atoms contain the same number of protons but different numbers of neutrons ...
... The atomic mass of each atom represents an average of all of the individual isotopes of that element. Two atoms contain the same number of protons but different numbers of neutrons ...
Chemistry - OnMyCalendar
... The atomic mass of each atom represents an average of all of the individual isotopes of that element. Two atoms contain the same number of protons but different numbers of neutrons ...
... The atomic mass of each atom represents an average of all of the individual isotopes of that element. Two atoms contain the same number of protons but different numbers of neutrons ...
(halogens group) 4-write down the electronic configuration
... …………to…………inside the same group. 4-the highest element in electro negativity is…….while the most metallic element is ……… 5- in the group , by increasing the atomic number , the electro negativity………… . 6-as the atomic number increases in the same period ,the non metallic property…………. 7-each period ...
... …………to…………inside the same group. 4-the highest element in electro negativity is…….while the most metallic element is ……… 5- in the group , by increasing the atomic number , the electro negativity………… . 6-as the atomic number increases in the same period ,the non metallic property…………. 7-each period ...
Periodic Trends Handout
... • Properties of elements repeat themselves periodically as they are placed in order by atomic number. Mendeleev’s Periodic Table (1869) • Arrange elements in order of increasing atomic mass. • Arrange elements in columns so that elements with similar properties are in the same group or family. • Ex: ...
... • Properties of elements repeat themselves periodically as they are placed in order by atomic number. Mendeleev’s Periodic Table (1869) • Arrange elements in order of increasing atomic mass. • Arrange elements in columns so that elements with similar properties are in the same group or family. • Ex: ...
File
... How did Mendeleev’s table make it possible for him to predict the properties of other, still undiscovered, elements? Mendeleev noted which families had spaces. He inferred that the missing elements would have properties similar to those of other members of the family. Two examples, gallium and germa ...
... How did Mendeleev’s table make it possible for him to predict the properties of other, still undiscovered, elements? Mendeleev noted which families had spaces. He inferred that the missing elements would have properties similar to those of other members of the family. Two examples, gallium and germa ...
Topic 5 - Holy Cross Collegiate
... How did Mendeleev’s table make it possible for him to predict the properties of other, still undiscovered, elements? Mendeleev noted which families had spaces. He inferred that the missing elements would have properties similar to those of other members of the family. Two examples, gallium and germa ...
... How did Mendeleev’s table make it possible for him to predict the properties of other, still undiscovered, elements? Mendeleev noted which families had spaces. He inferred that the missing elements would have properties similar to those of other members of the family. Two examples, gallium and germa ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... Science 2nd prep 1st term 1st lesson - He arranged elements in an ascending order according to their atomic numbers. - he added the inert gases in the (0) zero group. - He classified the elements of each group into two subgroups (A&B) as they differ in their ...
... Science 2nd prep 1st term 1st lesson - He arranged elements in an ascending order according to their atomic numbers. - he added the inert gases in the (0) zero group. - He classified the elements of each group into two subgroups (A&B) as they differ in their ...
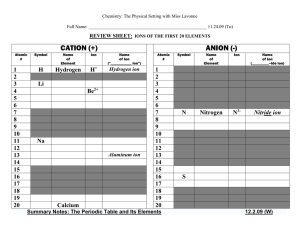
IONS OF THE FIRST 20 ELEMENTS
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
Ionization energy
... When placed in increasing order of their atomic masses, every eighth element showed similar physical and chemical properties. ...
... When placed in increasing order of their atomic masses, every eighth element showed similar physical and chemical properties. ...
for the quiz on 6 mar
... 19.98 In order to have a stronger acid, you want to make the O-H bond weaker. (There is a figure on p.646 in Ch. 15 which may help with this…) If you increase the number of oxygens in the molecule, you are drawing more electron density away from the Cl by bonding it to more (greater number of) elect ...
... 19.98 In order to have a stronger acid, you want to make the O-H bond weaker. (There is a figure on p.646 in Ch. 15 which may help with this…) If you increase the number of oxygens in the molecule, you are drawing more electron density away from the Cl by bonding it to more (greater number of) elect ...
The Periodic Table
... Development of the periodic table (cont) • Dimitri Mendeleev, in 1869, used this information and produced the first orderly arrangement of all 63 known elements. • He arranged them in a similar way to Newlands and created the first periodic table. • Mendeleev started a new row each time he noticed ...
... Development of the periodic table (cont) • Dimitri Mendeleev, in 1869, used this information and produced the first orderly arrangement of all 63 known elements. • He arranged them in a similar way to Newlands and created the first periodic table. • Mendeleev started a new row each time he noticed ...