Chemistry A- Periodic Table Packet
... members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. NEW: The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. Groups (vertical, “up and down”) – ...
... members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. NEW: The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. Groups (vertical, “up and down”) – ...
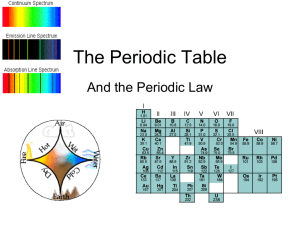
The Periodic Table
... • First ionization energies generally decrease as you move down a group. • With the valence electrons farther away from the nucleus, less energy is required to remove them. ...
... • First ionization energies generally decrease as you move down a group. • With the valence electrons farther away from the nucleus, less energy is required to remove them. ...
File
... how the trend moves across period and group on their own periodic table Effective nuclear charge The effective nuclear charge is the net positive charge experienced by an electron in a multielectron atom. The term "effective" is used because the shielding effect of negatively charged electrons preve ...
... how the trend moves across period and group on their own periodic table Effective nuclear charge The effective nuclear charge is the net positive charge experienced by an electron in a multielectron atom. The term "effective" is used because the shielding effect of negatively charged electrons preve ...
introduction-to-general-organic-and-biochemistry-10th
... the nucleus and electrons are added to the valence shell. For elements in the same period, the principal energy level remains the same (for example, the valence electrons of all second period elements occupy the second principal energy level). But in going from one element to the next across a perio ...
... the nucleus and electrons are added to the valence shell. For elements in the same period, the principal energy level remains the same (for example, the valence electrons of all second period elements occupy the second principal energy level). But in going from one element to the next across a perio ...
File - CToThe3Chemistry
... attracting) is further and further away. Ability to attract electrons increases as you move across periods, since the nucleus (and positive charge for attracting) is becoming more and more positive. ...
... attracting) is further and further away. Ability to attract electrons increases as you move across periods, since the nucleus (and positive charge for attracting) is becoming more and more positive. ...
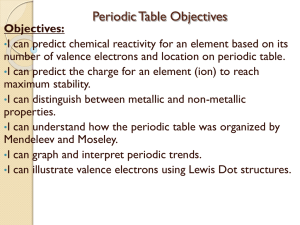
Objectives - Warren County Public Schools
... number of valence electrons and location on periodic table. •I can predict the charge for an element (ion) to reach maximum stability. •I can distinguish between metallic and non-metallic properties. •I can understand how the periodic table was organized by Mendeleev and Moseley. •I can graph and in ...
... number of valence electrons and location on periodic table. •I can predict the charge for an element (ion) to reach maximum stability. •I can distinguish between metallic and non-metallic properties. •I can understand how the periodic table was organized by Mendeleev and Moseley. •I can graph and in ...
Metal found in the salt
... – Families (groups) had similar chemical and physical properties – Discovered all elements in same family had same number of valence e- -outermost electrons in highest energy level – Why? ...
... – Families (groups) had similar chemical and physical properties – Discovered all elements in same family had same number of valence e- -outermost electrons in highest energy level – Why? ...
History of the Periodic Table
... Alkaline earth metals reactive metals Halogens most reactive nonmetals Noble gases don’t react ...
... Alkaline earth metals reactive metals Halogens most reactive nonmetals Noble gases don’t react ...
FREE Sample Here
... The atomic weight is 6.941 amu, which is nearer to 7 amu than 6 amu. Therefore, lithium7 is the more abundant isotope. The relative abundances for these two isotopes are 92.50 percent for lithium-7 and 7.50 percent for lithium-6. ...
... The atomic weight is 6.941 amu, which is nearer to 7 amu than 6 amu. Therefore, lithium7 is the more abundant isotope. The relative abundances for these two isotopes are 92.50 percent for lithium-7 and 7.50 percent for lithium-6. ...
Dmitri Mendeleev
... Mendeleev noticed that patterns appeared when the elements were arranged in order of increasing atomic mass. As he laid out cards, each element had properties similar to the elements above and below it. Mendeleev's table was not perfect, however. Arranging the elements by increasing atomic mass left ...
... Mendeleev noticed that patterns appeared when the elements were arranged in order of increasing atomic mass. As he laid out cards, each element had properties similar to the elements above and below it. Mendeleev's table was not perfect, however. Arranging the elements by increasing atomic mass left ...
Basic Chemistry Part 1 Presentation
... nucleus holding up to 2 electrons L or 2nd Shell – can hold up to a maximum of 8 electrons M or 3rd shell – can hold up to a maximum of 18 electrons ...
... nucleus holding up to 2 electrons L or 2nd Shell – can hold up to a maximum of 8 electrons M or 3rd shell – can hold up to a maximum of 18 electrons ...
1 Elements, atoms and electrons
... artificial radioactive isotopes such as carbon-14, iodine-131, nitrogen-15, oxygen-17, phosphorus-32, sulfur-35, tritium (hydrogen-3), iron-59, and sodium-24. There are a number of properties of radioisotopes that particularly lend them to a variety of uses in biology. 1. Radioisotopes can be detect ...
... artificial radioactive isotopes such as carbon-14, iodine-131, nitrogen-15, oxygen-17, phosphorus-32, sulfur-35, tritium (hydrogen-3), iron-59, and sodium-24. There are a number of properties of radioisotopes that particularly lend them to a variety of uses in biology. 1. Radioisotopes can be detect ...
Periodic Classification of Element (NCERT )
... (i) During the process of ionization, the electron to be removed from beryllium atom is a 2selectron, whereas the electron to be removed from boron atom is a 2p-electron. Now, 2selectrons are more strongly attached to the nucleus than 2p-electrons. Therefore, more energy is required to remove a 2s-e ...
... (i) During the process of ionization, the electron to be removed from beryllium atom is a 2selectron, whereas the electron to be removed from boron atom is a 2p-electron. Now, 2selectrons are more strongly attached to the nucleus than 2p-electrons. Therefore, more energy is required to remove a 2s-e ...
Trends of the Periodic Table - Old Saybrook Public Schools
... The means it will require more energy to remove the outer most electron. ...
... The means it will require more energy to remove the outer most electron. ...
The Structure of the Atom
... search for methods of separating metals from ores. E. Raison bun or plum pudding model. F. Earliest suggestion that matter was composed of atoms. G. Particles that have the same electronic configuration. H. Subatomic particle not found in the nucleus of the atom. I. The number of protons found in th ...
... search for methods of separating metals from ores. E. Raison bun or plum pudding model. F. Earliest suggestion that matter was composed of atoms. G. Particles that have the same electronic configuration. H. Subatomic particle not found in the nucleus of the atom. I. The number of protons found in th ...
Chemistry Primer for pH Measurements
... In molecules, atoms are held together by sharing electrons (covalent bonds). In order to maximize these bonds, the atoms adopt specific positions relative to each other, i.e. each molecule has its own definite geometric structure. For instance in the water molecule, the two hydrogen atoms are bonded ...
... In molecules, atoms are held together by sharing electrons (covalent bonds). In order to maximize these bonds, the atoms adopt specific positions relative to each other, i.e. each molecule has its own definite geometric structure. For instance in the water molecule, the two hydrogen atoms are bonded ...
atomic radii
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
atomic radii
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
periodic table - Cloudfront.net
... than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
... than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
Ch. 17 PPT
... than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
... than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
Student Exploration Sheet: Growing Plants
... people if there is an empty seat available? ________________________ Gizmo Warm-up Just like passengers getting on a bus, electrons orbit the nuclei of atoms in particular patterns. You will discover these patterns (and how electrons sometimes act like passengers boarding a bus) with the Electron Co ...
... people if there is an empty seat available? ________________________ Gizmo Warm-up Just like passengers getting on a bus, electrons orbit the nuclei of atoms in particular patterns. You will discover these patterns (and how electrons sometimes act like passengers boarding a bus) with the Electron Co ...
Summary of things you need to know from this first quarter. The
... 1. Each element is made up of tiny particles called atoms 2. The atoms of a given element are identical 3. Chemical compounds are formed when atoms combine with each other. A given compound always has the same relative numbers and types of atoms 4. Chemical reactions involve reorganizations of the a ...
... 1. Each element is made up of tiny particles called atoms 2. The atoms of a given element are identical 3. Chemical compounds are formed when atoms combine with each other. A given compound always has the same relative numbers and types of atoms 4. Chemical reactions involve reorganizations of the a ...
The Nuts and Bolts of Periodic Tables
... What's going on: Hardware stores need to organize their fasteners so that customers can easily find and purchase them. In the case of fasteners, they can be organize by their physical properties and by how they interact with one another, like how a specific bolt interacts with a specific nut. ...
... What's going on: Hardware stores need to organize their fasteners so that customers can easily find and purchase them. In the case of fasteners, they can be organize by their physical properties and by how they interact with one another, like how a specific bolt interacts with a specific nut. ...
CPO Science Link Teacher`s Guide
... On the far right on the periodic table is Group 18, the noble gases, including the elements helium (He), neon (Ne), and argon (Ar). These elements do not naturally form chemical bonds with other atoms. They are almost always found in their pure state, not as part of compounds. Elements in Groups 3 t ...
... On the far right on the periodic table is Group 18, the noble gases, including the elements helium (He), neon (Ne), and argon (Ar). These elements do not naturally form chemical bonds with other atoms. They are almost always found in their pure state, not as part of compounds. Elements in Groups 3 t ...