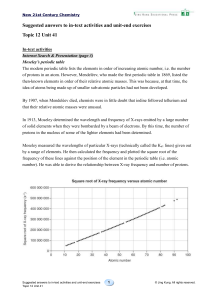
1.5 trends in the periodic table
... 9. Elements that are used for structural support of buildings, roads, vehicles, furniture, and so on should be unreactive — for example, metals that are resistant to the “rusting” reaction. Containers should be made of unreactive materials, especially in a chemistry lab or a kitchen. A match that do ...
... 9. Elements that are used for structural support of buildings, roads, vehicles, furniture, and so on should be unreactive — for example, metals that are resistant to the “rusting” reaction. Containers should be made of unreactive materials, especially in a chemistry lab or a kitchen. A match that do ...
Electron Configurations and Periodic Properties Name
... Objective: You have learned how the atoms are arranged and how to determine the electron configurations for atoms. Now we need to look at the relationship between electron configurations and periodic trends. For this assignment, you will need Modern Chemistry, pp.140-152 (You may skip the section on ...
... Objective: You have learned how the atoms are arranged and how to determine the electron configurations for atoms. Now we need to look at the relationship between electron configurations and periodic trends. For this assignment, you will need Modern Chemistry, pp.140-152 (You may skip the section on ...
Chapter 8
... y What is the periodic trend for ionization energy? y What is the difference between 1st and successive ionization energies? y ...
... y What is the periodic trend for ionization energy? y What is the difference between 1st and successive ionization energies? y ...
The Evolution of the Periodic System
... challenged by subsequent discoveries. One notable occasion arose in 1894, when William Ramsay of University College London and Lord Rayleigh ( John William Strutt) of the Royal Institution in London discovered the element argon; over the next few years, Ramsay announced the identification of four ot ...
... challenged by subsequent discoveries. One notable occasion arose in 1894, when William Ramsay of University College London and Lord Rayleigh ( John William Strutt) of the Royal Institution in London discovered the element argon; over the next few years, Ramsay announced the identification of four ot ...
The Evolution of the Periodic System - Science
... challenged by subsequent discoveries. One notable occasion arose in 1894, when William Ramsay of University College London and Lord Rayleigh ( John William Strutt) of the Royal Institution in London discovered the element argon; over the next few years, Ramsay announced the identification of four ot ...
... challenged by subsequent discoveries. One notable occasion arose in 1894, when William Ramsay of University College London and Lord Rayleigh ( John William Strutt) of the Royal Institution in London discovered the element argon; over the next few years, Ramsay announced the identification of four ot ...
Periodic Table
... shell but with increasing nuclear charge. The increasing number of protons (higher Z) attracts the electrons more, making it harder to remove an electron from the atom-hence a higher IE. Vertical: As you go down a group from top to bottom, you always have the same valence shell configuration. Howeve ...
... shell but with increasing nuclear charge. The increasing number of protons (higher Z) attracts the electrons more, making it harder to remove an electron from the atom-hence a higher IE. Vertical: As you go down a group from top to bottom, you always have the same valence shell configuration. Howeve ...
Chapter 5
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
Periodic Law
... electrons when it is chemically combined with another atom. • decreases as you move down a group • increases as you go across a period from left to right. ...
... electrons when it is chemically combined with another atom. • decreases as you move down a group • increases as you go across a period from left to right. ...
Unit 1: Introduction to Chemistry Directions: Use your notes
... you are unsure of an answer you can email me [email protected] or chat on Remind. Scientific Theories Dalton theorized that atoms were the smallest particle and could not be divided. Atoms can bond with one another in whole number ratios to form compounds but cannot be created or destroye ...
... you are unsure of an answer you can email me [email protected] or chat on Remind. Scientific Theories Dalton theorized that atoms were the smallest particle and could not be divided. Atoms can bond with one another in whole number ratios to form compounds but cannot be created or destroye ...
Bohr Model Practice PP
... protons and 8 electrons. Choice A has 9 electrons, making it an ion. Bonus questions: - Is this ion negatively or positively charged? - Which answer choice shows an isotope? ...
... protons and 8 electrons. Choice A has 9 electrons, making it an ion. Bonus questions: - Is this ion negatively or positively charged? - Which answer choice shows an isotope? ...
Electron Arrangements - Madison Public Schools
... 1. Atoms increase in size as you move down a group on the periodic table Why? • Outermost electrons are in higher energy levels. Orbital size increases with energy level. ...
... 1. Atoms increase in size as you move down a group on the periodic table Why? • Outermost electrons are in higher energy levels. Orbital size increases with energy level. ...
Periodic Table (Wiki)
... atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never se ...
... atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never se ...
Periodic Trends Review
... 1. Why did Mendeleyev leave spaces in his periodic table? Mendeleev left spaces in his periodic table because he predicted the existence of undiscovered elements. 2. What is the periodic law and how does is relate to the modern periodic table. The periodic law says that chemical similarities occur a ...
... 1. Why did Mendeleyev leave spaces in his periodic table? Mendeleev left spaces in his periodic table because he predicted the existence of undiscovered elements. 2. What is the periodic law and how does is relate to the modern periodic table. The periodic law says that chemical similarities occur a ...
4 PERIODIC TABLE AND ATOMIC PROPERTIES W
... (ii) The elements below the diagonal line and to the left are metals. (Hydrogen is a nonmetal and is an exception)The metallic character is more marked the farther an element is from the diagonal line and down. All lanthanoids and actinoids are metals. (iii) The elements along the diagonal line are ...
... (ii) The elements below the diagonal line and to the left are metals. (Hydrogen is a nonmetal and is an exception)The metallic character is more marked the farther an element is from the diagonal line and down. All lanthanoids and actinoids are metals. (iii) The elements along the diagonal line are ...
TOC 9/18 Organization of Periodic Table
... Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms. ...
... Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms. ...
Ex. 41 Answer
... The elements found by chemists before Seaborg were discovered — they existed on Earth already. The elements found by Seaborg were actually made — they are elements that do not exist naturally on Earth. Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a ...
... The elements found by chemists before Seaborg were discovered — they existed on Earth already. The elements found by Seaborg were actually made — they are elements that do not exist naturally on Earth. Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a ...
Trends in the Periodic Table_Notes
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
Periodic Trends
... From Xe, go 2 spaces across the s-block in the 6th row 6s2 Then detour to go 14 spaces across the f-block 4f14 note: for the f-block, n = row – 2 = 6 – 2 = 4 Finally go 9 spaces into the d-block on the 6th row 5d9 note: for the d-block, n = row – 1 = 6 – 1 = 5 ...
... From Xe, go 2 spaces across the s-block in the 6th row 6s2 Then detour to go 14 spaces across the f-block 4f14 note: for the f-block, n = row – 2 = 6 – 2 = 4 Finally go 9 spaces into the d-block on the 6th row 5d9 note: for the d-block, n = row – 1 = 6 – 1 = 5 ...
Section 2 Electron Configuration and the Periodic Table Chapter 5
... radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
... radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
The perfect K-12 presentation ever (replace this with your title)
... Main Sequence Stars, such as Red Giants, derive their energy from hydrogen fusion. ...
... Main Sequence Stars, such as Red Giants, derive their energy from hydrogen fusion. ...
Name
... additional elements were identified by using their line spectra and about 65 elements had been identified. A. J.W. Dobereiner: Organized the elements into ______________ with similar _____________. He called these groups ________________. The middle element is often the ____________________ of the o ...
... additional elements were identified by using their line spectra and about 65 elements had been identified. A. J.W. Dobereiner: Organized the elements into ______________ with similar _____________. He called these groups ________________. The middle element is often the ____________________ of the o ...
Inorganic and Physical Chemistry - university of nairobi staff profiles
... 5. Classify each of the following substances as an element or a compound: (a) hydrogen, (b) water, (c) gold, (d) sugar. 6. Classify each of the following as an element, a compound, a homogeneous mixture, or a heterogeneous mixture: (a) seawater, (b) helium gas, (c) sodium chloride (table salt), (d) ...
... 5. Classify each of the following substances as an element or a compound: (a) hydrogen, (b) water, (c) gold, (d) sugar. 6. Classify each of the following as an element, a compound, a homogeneous mixture, or a heterogeneous mixture: (a) seawater, (b) helium gas, (c) sodium chloride (table salt), (d) ...
Periodic Relationships Among the Elements
... • The presence of a partially filled d sublevels in a transition element results in colored compounds. • Elements with completely full or completely empty subshells are colorless, – For example Zinc which has a full d subshell. Its compounds are white ...
... • The presence of a partially filled d sublevels in a transition element results in colored compounds. • Elements with completely full or completely empty subshells are colorless, – For example Zinc which has a full d subshell. Its compounds are white ...
Atoms and Ions Practice Problems
... A) They are good conductors of heat. B) They are shiny. C) They are good conductors of electricity. D) They react vigorously with water. E) Most of them are liquids at room temperature. ...
... A) They are good conductors of heat. B) They are shiny. C) They are good conductors of electricity. D) They react vigorously with water. E) Most of them are liquids at room temperature. ...
Background Information: The periodic table is arranged in order of
... A group is a vertical column of the periodic table. Elements with similar physical and chemical properties belong in a group. The group number gives the number of valence electrons in an element. For example, the Alkali Metals are in Group 1 (one valence electron), and the Halogens are in Group 7 (7 ...
... A group is a vertical column of the periodic table. Elements with similar physical and chemical properties belong in a group. The group number gives the number of valence electrons in an element. For example, the Alkali Metals are in Group 1 (one valence electron), and the Halogens are in Group 7 (7 ...