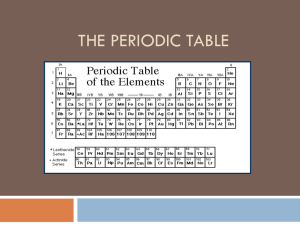
Organizing the periodic table
... which have similar characteristics. There are eighteen groups in the periodic table. The lanthanides and actinides do not fit in the periodic table because the table would be far to wide and are not given a group. ...
... which have similar characteristics. There are eighteen groups in the periodic table. The lanthanides and actinides do not fit in the periodic table because the table would be far to wide and are not given a group. ...
Title?
... In atoms of the Group 1A elements below, there is only one electron in the highest occupied energy level. ...
... In atoms of the Group 1A elements below, there is only one electron in the highest occupied energy level. ...
Trends of the Periodic
... Electronegativity is an atom's ability or desire to take another atom's electrons. Period - Electronegativity increases as you go from left to right across a period. Why? - Elements on the left (metals) of the period table have 1 -2 valence electrons and would rather give those few valence electron ...
... Electronegativity is an atom's ability or desire to take another atom's electrons. Period - Electronegativity increases as you go from left to right across a period. Why? - Elements on the left (metals) of the period table have 1 -2 valence electrons and would rather give those few valence electron ...
The Structure of the Atom
... A great deal of practical chemical knowledge was accumulated from their D. search for methods of separating metals from ores. E. Raison bun or plum pudding model. F. Earliest suggestion that matter was composed of atoms. G. Particles that have the same electronic configuration. H. Subatomic particle ...
... A great deal of practical chemical knowledge was accumulated from their D. search for methods of separating metals from ores. E. Raison bun or plum pudding model. F. Earliest suggestion that matter was composed of atoms. G. Particles that have the same electronic configuration. H. Subatomic particle ...
Chapter 8 Reading Guide Name: AP Chemistry 2016
... 24. Sketch a periodic table. Identify the location of atoms that are filling their last electrons in s, p, d, and f orbitals. ...
... 24. Sketch a periodic table. Identify the location of atoms that are filling their last electrons in s, p, d, and f orbitals. ...
UNIT-3 Classification of elements and periodicity
... 18. The position of helium is in 18th group of p block and not in 2nd group of s block of long form of periodic table. Justify the statement. 19. To which block of the periodic table do the elements of group-I and II belong? 20. How many groups of elements form p block of the periodic table? 21. Wha ...
... 18. The position of helium is in 18th group of p block and not in 2nd group of s block of long form of periodic table. Justify the statement. 19. To which block of the periodic table do the elements of group-I and II belong? 20. How many groups of elements form p block of the periodic table? 21. Wha ...
Atoms
... • Cations are smaller than their corresponding atom. Why? • Loss of electrons means the positive nucleus has a greater attraction on the remaining electrons • Anions are larger than their corresponding atom. Why? • Gain of electrons means the nucleus has less attraction for the electrons as well as ...
... • Cations are smaller than their corresponding atom. Why? • Loss of electrons means the positive nucleus has a greater attraction on the remaining electrons • Anions are larger than their corresponding atom. Why? • Gain of electrons means the nucleus has less attraction for the electrons as well as ...
The Periodic Table of Elements
... This makes them very reactive because they are SO CLOSE to having an octet, just need 1 more e- ...
... This makes them very reactive because they are SO CLOSE to having an octet, just need 1 more e- ...
Chapter 8 Electron Configurations and Periodicity
... elements by increasing atomic mass and with similar properties in columns. In some places, there were missing elements whose properties he predicted. When gallium, scandium, and germanium were isolated and characterized, their properties were almost identical to those predicted by Mendeleev for eka- ...
... elements by increasing atomic mass and with similar properties in columns. In some places, there were missing elements whose properties he predicted. When gallium, scandium, and germanium were isolated and characterized, their properties were almost identical to those predicted by Mendeleev for eka- ...
atomic radii
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
atomic radii
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
... and p orbitals that were farther and farther away from the nucleus, IE is affected in the same way, leading to an easier removal for electrons that lie in the outermost, most distant s and p orbitals. • also, you must realize that electrons that lie in between the nucleus and those outermost s and p ...
File
... Properties of Oxides Across a Period Metallic characters of the elements decreases from left to right across a Period and increases from top to bottom within a Group. Most oxides of representative metals are ionic compounds; in water they act as bases, producing OH ions and reacting with acids. Ex ...
... Properties of Oxides Across a Period Metallic characters of the elements decreases from left to right across a Period and increases from top to bottom within a Group. Most oxides of representative metals are ionic compounds; in water they act as bases, producing OH ions and reacting with acids. Ex ...
Isotopes and the Electron Configuration of the Blocks in the Periodic
... It is known that elements of the Periodic Table of Elements have fractional numerical values of atomic masses. This is because the elements consists of, as regularly, a mix of inborn (native) isotopes. For this reason we conclude that the average weighted atomic mass of all stable isotopes of any el ...
... It is known that elements of the Periodic Table of Elements have fractional numerical values of atomic masses. This is because the elements consists of, as regularly, a mix of inborn (native) isotopes. For this reason we conclude that the average weighted atomic mass of all stable isotopes of any el ...
ELECTRONS IN THE ATOM
... The electron configuration actually gives us the location of any element on the periodic table. We simply have to be able to count as we fill in boxes! The way we read the configuration is to account for every electron in the atom – time to remember that as elements progress across the periodic tabl ...
... The electron configuration actually gives us the location of any element on the periodic table. We simply have to be able to count as we fill in boxes! The way we read the configuration is to account for every electron in the atom – time to remember that as elements progress across the periodic tabl ...
Student Exploration Sheet: Growing Plants
... 9. Practice: In the spaces below, write electron configurations for the next four elements: nitrogen, oxygen, fluorine, and neon. When you are finished, use the Gizmo to check your work. Correct any improper configurations. 1s Nitrogen configuration: ___________________ ...
... 9. Practice: In the spaces below, write electron configurations for the next four elements: nitrogen, oxygen, fluorine, and neon. When you are finished, use the Gizmo to check your work. Correct any improper configurations. 1s Nitrogen configuration: ___________________ ...
chemistry, grade 11, university preparation, sch3u
... Never try to push glass tubing through a cork or rubber stopper without knowing exactly how to proceed—use glycerine to wet the glass and stopper. Be very careful with scalpels and razors—never wander around the lab with an open blade in your hand. Cutting instruments should be kept really sharp—a b ...
... Never try to push glass tubing through a cork or rubber stopper without knowing exactly how to proceed—use glycerine to wet the glass and stopper. Be very careful with scalpels and razors—never wander around the lab with an open blade in your hand. Cutting instruments should be kept really sharp—a b ...
CHAPTER 14 Chemical Periodicity
... (inert) element. The representative elements - Group A elements - have their s and p orbitals being filled. These include : o Group 1A - Li, Na, K etc. - all very reactive with one electron in the outer s orbital o Group 2A - Be, Mg, Ca etc. - all quite reactive with 2 electrons filling their outer ...
... (inert) element. The representative elements - Group A elements - have their s and p orbitals being filled. These include : o Group 1A - Li, Na, K etc. - all very reactive with one electron in the outer s orbital o Group 2A - Be, Mg, Ca etc. - all quite reactive with 2 electrons filling their outer ...
D. - Taylor County Schools
... • The two rows under the periodic table are called the inner transition metals. ...
... • The two rows under the periodic table are called the inner transition metals. ...
Chapter 5.3 Periodic Properties
... Consider two main-group elements, A and B. Element A has a first ionization energy of 419 kJ/mol. Element B has a first ionization energy of 1000 kJ/mol. Decide if each element is more likely to be in the s block or p block. Which element is more likely to form a positive ion ...
... Consider two main-group elements, A and B. Element A has a first ionization energy of 419 kJ/mol. Element B has a first ionization energy of 1000 kJ/mol. Decide if each element is more likely to be in the s block or p block. Which element is more likely to form a positive ion ...
Periodic Table
... gave off x-rays with distinct wavelengths. Essentially what was happening was that the cathode rays (high energy electrons) were knocking out the inner-most electrons of the metal targets. X-rays were emitted when an outer electron "fell" down into this inner shell. Since the inner-most electrons ar ...
... gave off x-rays with distinct wavelengths. Essentially what was happening was that the cathode rays (high energy electrons) were knocking out the inner-most electrons of the metal targets. X-rays were emitted when an outer electron "fell" down into this inner shell. Since the inner-most electrons ar ...
The Periodic Table and Periodic Law
... What does a group tell us about valence electrons and ion formation? ...
... What does a group tell us about valence electrons and ion formation? ...
Discovering Elements
... directly to the filling of the shells. They give the table its periodic nature, as elements with similar electron configurations fall into columns called groups. Group 1, the alkali metals (Li, Na, K, Rb, Cs, Fr), all have 1 outer electron, and Group 7, the halogens (F, Cl, Br, I, At), all have 7 oute ...
... directly to the filling of the shells. They give the table its periodic nature, as elements with similar electron configurations fall into columns called groups. Group 1, the alkali metals (Li, Na, K, Rb, Cs, Fr), all have 1 outer electron, and Group 7, the halogens (F, Cl, Br, I, At), all have 7 oute ...
Periodic Table Packet
... ii. produce covalent bonds by sharing electrons with other nonmetals b. Physical properties i. exist as gases, molecular solids, or network solids at room temperature except bromine ii. solids are brittle - not ductile or malleable iii. solids are dull - do not reflect light even when polished iv. p ...
... ii. produce covalent bonds by sharing electrons with other nonmetals b. Physical properties i. exist as gases, molecular solids, or network solids at room temperature except bromine ii. solids are brittle - not ductile or malleable iii. solids are dull - do not reflect light even when polished iv. p ...
Periodicity - ilc.edu.hk
... are great ∵ extent of bond breaking : boiling >> melting Particles are completely separated on boiling For Gp4A elements, the differences between m.p. and b.p. are relatively small ∵ extent of bond breaking : boiling melting ...
... are great ∵ extent of bond breaking : boiling >> melting Particles are completely separated on boiling For Gp4A elements, the differences between m.p. and b.p. are relatively small ∵ extent of bond breaking : boiling melting ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.