Chemistry lecture notes
... Isotopes have the same atomic number (same number of protons), but a different atomic mass number (a different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional ...
... Isotopes have the same atomic number (same number of protons), but a different atomic mass number (a different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional ...
Name Test Review Chemistry Unit 2: The Atom 1. Fill in the blank
... 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the time allowed for shipment is 15 days, how much mercury-197 will need to be ordered? 24. How much strontium-90 will remain after 4 half-lives if the initial am ...
... 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the time allowed for shipment is 15 days, how much mercury-197 will need to be ordered? 24. How much strontium-90 will remain after 4 half-lives if the initial am ...
Chapter 6 Review“The Periodic Table”
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
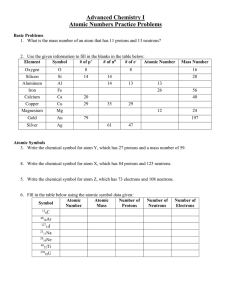
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Salesian High School Elements and atoms Chemistry quiz The
... b) Elements that have the same mass number but different atomic numbers c) Atoms that have the same number of protons but a different number of neutrons d) Elements that have the same number of neutrons but different mass numbers ...
... b) Elements that have the same mass number but different atomic numbers c) Atoms that have the same number of protons but a different number of neutrons d) Elements that have the same number of neutrons but different mass numbers ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... 22. If the half-life for the radioactive decay of zirconium-84 is 26 minutes and I start with a 175 gram sample, how much will be left over after 104 minutes? 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the ...
... 22. If the half-life for the radioactive decay of zirconium-84 is 26 minutes and I start with a 175 gram sample, how much will be left over after 104 minutes? 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the ...
Advanced Chemistry Midterm
... 23. What are the electronegativity difference ranges for nonpolar bonds? For polar bonds? For ionic bonds? ...
... 23. What are the electronegativity difference ranges for nonpolar bonds? For polar bonds? For ionic bonds? ...
Chapter 3 Chemical Foundations
... atomic number (Z) = mass number (A) = element symbol (X) = Note: mass number= Therefore …. mass number = ……. A= Z + number of neutrons ….. Number of neutrons = A-Z Note: For any given element on the periodic table: Number of protons = In order to symbolically represent elements and isotopes chemists ...
... atomic number (Z) = mass number (A) = element symbol (X) = Note: mass number= Therefore …. mass number = ……. A= Z + number of neutrons ….. Number of neutrons = A-Z Note: For any given element on the periodic table: Number of protons = In order to symbolically represent elements and isotopes chemists ...
Chap 7: Around the Room Review
... 9. Who developed the first periodic table of the elements? 10. The elements in Group 1 are commonly called the _______. 11. The isotope nitrogen-13 has a half-life of 10 minutes. If you start with 40 grams, how many grams of this isotope will you have remaining after 30 minutes? Show your work. 12. ...
... 9. Who developed the first periodic table of the elements? 10. The elements in Group 1 are commonly called the _______. 11. The isotope nitrogen-13 has a half-life of 10 minutes. If you start with 40 grams, how many grams of this isotope will you have remaining after 30 minutes? Show your work. 12. ...
22-Introduction to Radioactivity
... conducting an experiment that models what happens during radioactive decay. Reviewing Some Ideas about Atoms Use your knowledge and information from the textbook to print the best word(s) in each sentence. 1. Atoms are the “basic” particles of chemical ______________________________________. 2. Ther ...
... conducting an experiment that models what happens during radioactive decay. Reviewing Some Ideas about Atoms Use your knowledge and information from the textbook to print the best word(s) in each sentence. 1. Atoms are the “basic” particles of chemical ______________________________________. 2. Ther ...
Atom Structure and Isotopes
... 1) What holds atoms together? an attraction of opposite charges 2) The atom is mostly empty space. ...
... 1) What holds atoms together? an attraction of opposite charges 2) The atom is mostly empty space. ...
Practice Test #2 - smhs
... The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abundant, what is the percent abundance of the heaviest isotope? The average atomic mass ...
... The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abundant, what is the percent abundance of the heaviest isotope? The average atomic mass ...
Practice Test #2 - smhs
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
Summative Assessment Study Guide Name: Due date: SPS1
... SPS3. Students will distinguish the characteristics and components of radioactivity. a. Differentiate among alpha and beta particles and gamma radiation. b. Differentiate between fission and fusion. c. Explain the process half-life as related to radioactive decay. d. Describe nuclear energy, its pra ...
... SPS3. Students will distinguish the characteristics and components of radioactivity. a. Differentiate among alpha and beta particles and gamma radiation. b. Differentiate between fission and fusion. c. Explain the process half-life as related to radioactive decay. d. Describe nuclear energy, its pra ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
The study of biology can help you better understand human
... Atoms of isotopes of an element have different number of protons The nucleus of an atom has positive charge. Atoms are mostly empty space. ...
... Atoms of isotopes of an element have different number of protons The nucleus of an atom has positive charge. Atoms are mostly empty space. ...
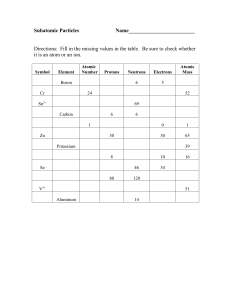
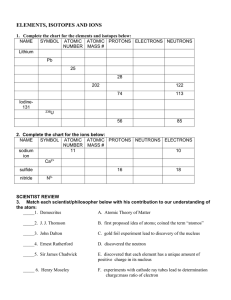
ELEMENTS, ISOTOPES AND IONS
... 1. Complete the chart for the elements and isotopes below: NAME SYMBOL ATOMIC ATOMIC PROTONS ELECTRONS NEUTRONS NUMBER MASS # ...
... 1. Complete the chart for the elements and isotopes below: NAME SYMBOL ATOMIC ATOMIC PROTONS ELECTRONS NEUTRONS NUMBER MASS # ...
BellWork 2/16/2015
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
Chapter 21 Powerpoint: Nuclear Chemistry
... The half-life of mercury-195 is 31 hours. If you start with a sample of 5.00 g, how much of it will still be left after 93 hours? ...
... The half-life of mercury-195 is 31 hours. If you start with a sample of 5.00 g, how much of it will still be left after 93 hours? ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.