How Atoms Differ Elements, Isotopes, and Ions
... Atoms are arranged in order by their atomic #. ...
... Atoms are arranged in order by their atomic #. ...
ch2_objectives
... 9. Define the terms energy and potential energy. Explain why electrons in the first electron shell have less potential energy than electrons in higher electron shells. 10. Distinguish among nonpolar covalent, polar covalent and ionic bonds. 11. Explain why strong covalent bonds and weak bonds are b ...
... 9. Define the terms energy and potential energy. Explain why electrons in the first electron shell have less potential energy than electrons in higher electron shells. 10. Distinguish among nonpolar covalent, polar covalent and ionic bonds. 11. Explain why strong covalent bonds and weak bonds are b ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 5. In Rutherford's Atomic model, the protons - positively charged particles are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The ...
... 5. In Rutherford's Atomic model, the protons - positively charged particles are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The ...
Atomic Number, Mass Number, and Isotopes
... ATOMS: All atoms of the same element have the same number of protons: the number of protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Alt ...
... ATOMS: All atoms of the same element have the same number of protons: the number of protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Alt ...
1st Term Review
... 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron configuration of a neutral calcium atom? 18. Atomic ...
... 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron configuration of a neutral calcium atom? 18. Atomic ...
Nuclear Particles p. 706
... (From Cobalt-60 and Cesium-137) & Research P32 and S35 Tracers: radioactive isotopes, natural and manufactured. Chemical tracers follow certain metabolic processes in the body. Irradiation: radioisotopes used to treat food so that it can be stored without refrigeration for a long time. ...
... (From Cobalt-60 and Cesium-137) & Research P32 and S35 Tracers: radioactive isotopes, natural and manufactured. Chemical tracers follow certain metabolic processes in the body. Irradiation: radioisotopes used to treat food so that it can be stored without refrigeration for a long time. ...
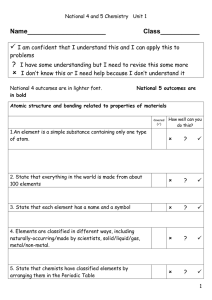
Properties of matter student notes[1]
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
Vocabulary and Section Summary
... Name ______________________________ Class___________________Date__________________ ...
... Name ______________________________ Class___________________Date__________________ ...
Periodic Table
... broken and formed Atoms remain unchanged, but the may be rearranged Involve only valence electrons Have small energy changes Reaction rates are influenced by temperature, pressure, concentration, and catalysts ...
... broken and formed Atoms remain unchanged, but the may be rearranged Involve only valence electrons Have small energy changes Reaction rates are influenced by temperature, pressure, concentration, and catalysts ...
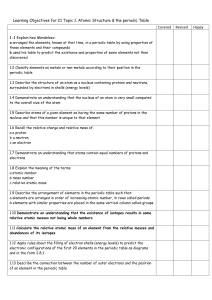
C2- Topic 1: Atomic structure and the periodic table. Assessable
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
C2 Topic 1 Can Do Sheet
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
(null): 096.AtomReview
... c. Electrons jump back down to specific “floors” and emit light with specific energy = specific color d. Color of light allows us to calculate energies of electrons WITHOUT BEING ABLE TO “SEE” THEM 3. Show spectrum of zinc atom: a. Each specific color tells us about the structure of specific electro ...
... c. Electrons jump back down to specific “floors” and emit light with specific energy = specific color d. Color of light allows us to calculate energies of electrons WITHOUT BEING ABLE TO “SEE” THEM 3. Show spectrum of zinc atom: a. Each specific color tells us about the structure of specific electro ...
HW-1-Ch1-Atomic-structure-W16
... 30. Nodes in a 4d orbital: a) Total nodes = b) Radial nodes = c) Angular nodes = ...
... 30. Nodes in a 4d orbital: a) Total nodes = b) Radial nodes = c) Angular nodes = ...
Chemical Bonding
... • The subatomic particles that make up atoms are protons, neutrons, and electrons. • Protons=Positive charge • Neutrons=Neutral charge • Electrons=Negative charge ...
... • The subatomic particles that make up atoms are protons, neutrons, and electrons. • Protons=Positive charge • Neutrons=Neutral charge • Electrons=Negative charge ...
Chapter 5
... masses 106.9041 amu and 108.9047 amu, respectively. The first isotope represents 51.82% and the second represents 48.18%. Determine the average atomic mass of silver. ...
... masses 106.9041 amu and 108.9047 amu, respectively. The first isotope represents 51.82% and the second represents 48.18%. Determine the average atomic mass of silver. ...
Chapter 18 Notes
... Proton- P+ positive charged - in nucleus Number of P+ distinguishes one atom from another Made of 2 up quarks (+2/3 charge) and 1 down ...
... Proton- P+ positive charged - in nucleus Number of P+ distinguishes one atom from another Made of 2 up quarks (+2/3 charge) and 1 down ...
The Nuclear Atom
... Democritus (460 B.C. – 370 B.C.) • first to suggest the existence of “atoms” ...
... Democritus (460 B.C. – 370 B.C.) • first to suggest the existence of “atoms” ...
L.O.
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
Word - chemmybear.com
... 2 Atoms and Elements P R A C T I C E 1. Certain properties are characteristic of metals. Which property means that you can pound the ...
... 2 Atoms and Elements P R A C T I C E 1. Certain properties are characteristic of metals. Which property means that you can pound the ...
PS.Ch6.Test.95
... 2 Atoms and Elements P R A C T I C E 1. Certain properties are characteristic of metals. Which property means that you can pound the ...
... 2 Atoms and Elements P R A C T I C E 1. Certain properties are characteristic of metals. Which property means that you can pound the ...
Kentucky newspapers 1949 look at the city, part 5
... counters and similar instruments for checking on the amount of radioactivity in the air of the pile building. When there is too much around for human safety, the Geigers click madly, measuring the gamma and beta rays. Geigers aren’t so good on detecting alpha rays, but other devices take care of tha ...
... counters and similar instruments for checking on the amount of radioactivity in the air of the pile building. When there is too much around for human safety, the Geigers click madly, measuring the gamma and beta rays. Geigers aren’t so good on detecting alpha rays, but other devices take care of tha ...
VOCABULARY name, date, hour: Fill in the number of each term
... ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons __ ...
... ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons __ ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.





![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)