Atomic Structure
... Early Atomic Theory “Cosmic substance is made up of an infinite number of elements or particles ...
... Early Atomic Theory “Cosmic substance is made up of an infinite number of elements or particles ...
Answer Key
... C. Word Problems: Be sure to show the formula you use and all your work for any calculations. No credit will be given for problems that do not show your work. 1. There are only two naturally occurring nuclides of gallium: gallium-69 (68.925 581 u) and gallium-71 (70.924 705 u). If the relative natu ...
... C. Word Problems: Be sure to show the formula you use and all your work for any calculations. No credit will be given for problems that do not show your work. 1. There are only two naturally occurring nuclides of gallium: gallium-69 (68.925 581 u) and gallium-71 (70.924 705 u). If the relative natu ...
Atom The smallest piece of matter that still has the properties of the
... A table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure and similar chemical properties appear in vertical columns. ...
... A table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure and similar chemical properties appear in vertical columns. ...
Test Review: Unit 1 - Ms. Hill`s Pre
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
Chapter 3 Atoms and Elements
... Since atomic mass is equal to the sum of the number of protons and neutrons, how can you have a fractional number? How was an atomic mass value of 35.45 arrived at? Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defi ...
... Since atomic mass is equal to the sum of the number of protons and neutrons, how can you have a fractional number? How was an atomic mass value of 35.45 arrived at? Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defi ...
Week 1 Grade 7 Thursday
... Atomic number = number of protons Atomic number = number of electrons in a neutral atom (not an ion) Atomic weight - atomic number = number of neutrons Isotopes have different numbers of neutrons, H normally has 0 neutrons ...
... Atomic number = number of protons Atomic number = number of electrons in a neutral atom (not an ion) Atomic weight - atomic number = number of neutrons Isotopes have different numbers of neutrons, H normally has 0 neutrons ...
A or `Mass Number` - Uplift Pinnacle Prep
... The US mint estimates that of all the pennies currently in circulation 66.5% of them are “new” (post-1982) pennies and 33.5% are ‘old’ pennies. A ‘new’ penny weighs 2.5g and an old penny weighs 3.1 g. Use this information to determine the average mass of a penny. ...
... The US mint estimates that of all the pennies currently in circulation 66.5% of them are “new” (post-1982) pennies and 33.5% are ‘old’ pennies. A ‘new’ penny weighs 2.5g and an old penny weighs 3.1 g. Use this information to determine the average mass of a penny. ...
File
... Each element has many possible mass numbers, but the most commonly occurring mass number is determined by rounding the atomic mass to the nearest whole number ...
... Each element has many possible mass numbers, but the most commonly occurring mass number is determined by rounding the atomic mass to the nearest whole number ...
Atomic Structure
... the same element. This means they will be used by cells to make compounds in the same way as non-radioactive isotopes. However, the radioactive isotopes are easily detected and this makes them useful as a “medical tracer”. They can be used to track the movement or accumulation of a particular chemic ...
... the same element. This means they will be used by cells to make compounds in the same way as non-radioactive isotopes. However, the radioactive isotopes are easily detected and this makes them useful as a “medical tracer”. They can be used to track the movement or accumulation of a particular chemic ...
Isotope PPT - MrsPage.com
... are called valence electrons Valence electrons are responsible the for the reactivity of an atom. ...
... are called valence electrons Valence electrons are responsible the for the reactivity of an atom. ...
SS18A - Atoms, Isotopes and Ions
... Since silver has an atomic mass of 107.87, this means that most of the stable isotopes that exist have a mass number of 108. In other words, the most common silver isotope is “silver-108.” To figure out the most common isotope for an element, round the atomic mass to the nearest whole number. 1. Loo ...
... Since silver has an atomic mass of 107.87, this means that most of the stable isotopes that exist have a mass number of 108. In other words, the most common silver isotope is “silver-108.” To figure out the most common isotope for an element, round the atomic mass to the nearest whole number. 1. Loo ...
atomic structure - IGCSE STUDY BANK
... There are small physical differences between the isotopes eg the heavier isotope has a greater density or boiling point. However, because they have the same number of protons they have the same electronic structure and identical chemically. Examples are illustrated below. Do NOT assume the word isot ...
... There are small physical differences between the isotopes eg the heavier isotope has a greater density or boiling point. However, because they have the same number of protons they have the same electronic structure and identical chemically. Examples are illustrated below. Do NOT assume the word isot ...
Isotope Practice Worksheet
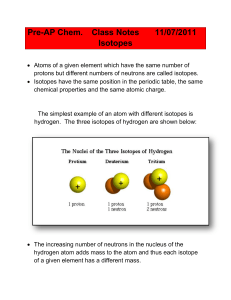
... For the isotopes of hydrogen: o 1H (or hydrogen-1), protium (usually just referred to as hydrogen) o 2H (or hydrogen-2), deuterium o 3H (or hydrogen-3), tritium, respectively Most of the light elements contain different proportions of at least two isotopes. Usually one isotope is the predomina ...
... For the isotopes of hydrogen: o 1H (or hydrogen-1), protium (usually just referred to as hydrogen) o 2H (or hydrogen-2), deuterium o 3H (or hydrogen-3), tritium, respectively Most of the light elements contain different proportions of at least two isotopes. Usually one isotope is the predomina ...
Chapter 4: The Structure of the Atom Early Ideas about Matter Name
... Oil drop experiment (gravity, e– charge, and charged plates) α – particle / gold foil ...
... Oil drop experiment (gravity, e– charge, and charged plates) α – particle / gold foil ...
isotopes notes
... • Neutrons were the last subatomic particles to be discovered because they have no electrical charge. ...
... • Neutrons were the last subatomic particles to be discovered because they have no electrical charge. ...
File
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
Atomic Structure
... • According to this model, the nucleus is tiny and densely packed compared with the atom as a whole. • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
... • According to this model, the nucleus is tiny and densely packed compared with the atom as a whole. • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
Chapter 18
... same element that have different numbers of neutrons • Average atomic mass—the weighted average mass of an element’s mixture of isotopes (used because most elements have more than one isotope) ...
... same element that have different numbers of neutrons • Average atomic mass—the weighted average mass of an element’s mixture of isotopes (used because most elements have more than one isotope) ...
File
... line. Electrons occupy the VOLUME, protons and neutrons constitute the MASS of an atom. ...
... line. Electrons occupy the VOLUME, protons and neutrons constitute the MASS of an atom. ...
3-10 What are isotopes?
... reason for this? ____________________________________________________________________________ __________________________________________________________________________________________ 5. According to the table, how are isotopes named? ______________________________________________ 6. What is true a ...
... reason for this? ____________________________________________________________________________ __________________________________________________________________________________________ 5. According to the table, how are isotopes named? ______________________________________________ 6. What is true a ...
The Basics of Atomic Structure
... • An Isotope is an atom with the same number of protons but a different number of neutrons; therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. • An Ion is an element with a number of electrons that d ...
... • An Isotope is an atom with the same number of protons but a different number of neutrons; therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. • An Ion is an element with a number of electrons that d ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.