Atoms and Atomic Theory
... This does not mean that there are 17 protons, 17 electrons and 18.5 neutrons in an atom of chlorine. It is not possible to have a fraction of a neutron, there can only be a whole number of neutrons in an atom. So what does it mean, and where does the 0.5 come from? Here is the explanation. The non i ...
... This does not mean that there are 17 protons, 17 electrons and 18.5 neutrons in an atom of chlorine. It is not possible to have a fraction of a neutron, there can only be a whole number of neutrons in an atom. So what does it mean, and where does the 0.5 come from? Here is the explanation. The non i ...
Metals
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
Chapter 4.3: How Atoms Differ
... Spontaneous process of ____________ nuclei emitting ___________ is called ______________ ___________. ...
... Spontaneous process of ____________ nuclei emitting ___________ is called ______________ ___________. ...
Name
... Atoms of different elements can _________________mix together or ___________________ combine. Chemical reactions occur when atoms are__________________, _________________, or _________________________. ...
... Atoms of different elements can _________________mix together or ___________________ combine. Chemical reactions occur when atoms are__________________, _________________, or _________________________. ...
Chapter 4 Review Worksheet
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
are made up of
... Severalscientists,including Newlands, Meyer, and Mendeleevworked on classificationsystems that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron a ...
... Severalscientists,including Newlands, Meyer, and Mendeleevworked on classificationsystems that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron a ...
Extra Credit Test Review
... 8. a) What is the difference between groups and periods on the Periodic table? _______________________________________________________________________ _______________________________________________________________________ b) Fill in the chart below. ...
... 8. a) What is the difference between groups and periods on the Periodic table? _______________________________________________________________________ _______________________________________________________________________ b) Fill in the chart below. ...
Atomic Number
... related to the atom were completed by -The nucleus is very _________________ Rutherford ______________ -His work was completed in__________ -The atom is mainly _______________ -________________________________ space James Chadwick Did work in __________ -Discovered the _________________, a Theorized ...
... related to the atom were completed by -The nucleus is very _________________ Rutherford ______________ -His work was completed in__________ -The atom is mainly _______________ -________________________________ space James Chadwick Did work in __________ -Discovered the _________________, a Theorized ...
Chapter 1
... Atoms of the same element can have different numbers of neutrons; the different possible versions of each element are called isotopes. For example, the most common isotope of hydrogen has no neutrons at all; there's also a hydrogen isotope called deuterium, with one neutron, and another, tritium, wi ...
... Atoms of the same element can have different numbers of neutrons; the different possible versions of each element are called isotopes. For example, the most common isotope of hydrogen has no neutrons at all; there's also a hydrogen isotope called deuterium, with one neutron, and another, tritium, wi ...
Chapter 7
... to explain why materials combined in definite proportions instead of random proportions. When hydrogen and oxygen are mixed, for example, only a certain number of grams of hydrogen will combine with any particular amount of oxygen to produce water. It was not until the 1980’s that atoms could be see ...
... to explain why materials combined in definite proportions instead of random proportions. When hydrogen and oxygen are mixed, for example, only a certain number of grams of hydrogen will combine with any particular amount of oxygen to produce water. It was not until the 1980’s that atoms could be see ...
Chapter 4 Structure of the Atom An atom is the smallest particle of an
... Democritus is the first person to define an atom. Greek philosopher 2000 years ago. Atom is so small and indestructible that it cannot be divided up into anything smaller. Atom means indivisible in Greek. Law of conservation of mass – ...
... Democritus is the first person to define an atom. Greek philosopher 2000 years ago. Atom is so small and indestructible that it cannot be divided up into anything smaller. Atom means indivisible in Greek. Law of conservation of mass – ...
Atomic Review
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
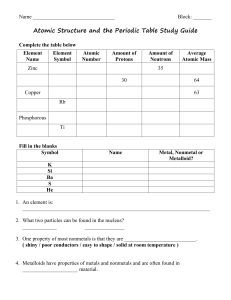
Element Symbol Number of Protons Number of electrons Number of
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
... 7. In the first diagram, draw in what he expected to happen 8. In the second diagram draw in what happened. ...
Average Atomic Mass 1213
... carbon--12/carbon carbon 12/carbon--14 or they man--made like can be man Einsteinium--252/Einsteinium Einsteinium 252/Einsteinium-251 Isotopes can be stable or unstable. Unstable isotopes will decay and are radioactive. ...
... carbon--12/carbon carbon 12/carbon--14 or they man--made like can be man Einsteinium--252/Einsteinium Einsteinium 252/Einsteinium-251 Isotopes can be stable or unstable. Unstable isotopes will decay and are radioactive. ...
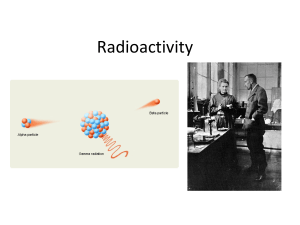
Chapter 12 –Radioactivity
... • Most of the isotopes which occur naturally are stable. • A few naturally occurring isotopes and all of the man-made isotopes are unstable. • Unstable isotopes can become stable by releasing different types of particles. • This process is called radioactive decay and the elements which undergo this ...
... • Most of the isotopes which occur naturally are stable. • A few naturally occurring isotopes and all of the man-made isotopes are unstable. • Unstable isotopes can become stable by releasing different types of particles. • This process is called radioactive decay and the elements which undergo this ...
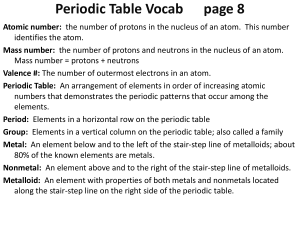
Periodic Table Vocab page 7
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
Radioactivity2015
... • Radioisotopes decay from one element to another until they are transformed into stable, non-radioactive isotopes. • For example, 238U decays 11 times, shedding mass and energy each time, eventually becoming 206Pb, a stable isotope. • Radioactive decay is spontaneous – it does not require an input ...
... • Radioisotopes decay from one element to another until they are transformed into stable, non-radioactive isotopes. • For example, 238U decays 11 times, shedding mass and energy each time, eventually becoming 206Pb, a stable isotope. • Radioactive decay is spontaneous – it does not require an input ...
The average atomic mass of an element is the sum of the
... the natural abundance of various isotopes of an element, it is simple to calculate the average atomic mass. For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4; therefore, the average atomic mass is very close to 4 amu (4.002602 amu). Chlorine consists ...
... the natural abundance of various isotopes of an element, it is simple to calculate the average atomic mass. For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4; therefore, the average atomic mass is very close to 4 amu (4.002602 amu). Chlorine consists ...
UNIT 1 - Grafton Public Schools
... What are the three kinds of subatomic particles? What makes one element different from another? How do isotopes of an element differ? How do you calculate the atomic mass of an element? How do nuclear reactions differ from chemical reactions? What are the three types of nuclear radiation? How much o ...
... What are the three kinds of subatomic particles? What makes one element different from another? How do isotopes of an element differ? How do you calculate the atomic mass of an element? How do nuclear reactions differ from chemical reactions? What are the three types of nuclear radiation? How much o ...
and View
... atom. * Number never changes* b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
... atom. * Number never changes* b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
The average atomic mass of an element is the sum of the
... The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance (the decimal associated with percent of atoms of that element that are of a given isotope). The average atomic mass of an element can be found on the periodic table, typically und ...
... The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance (the decimal associated with percent of atoms of that element that are of a given isotope). The average atomic mass of an element can be found on the periodic table, typically und ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.