Atom Review
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
Atomic Structure Subatomic Particles Atoms are made up of even
... determined relative to the mass of carbon-12, which by definition is exactly 12 amu. The atomic mass of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. A weighted average mass reflects both the mass and the relative abundance of the isotopes as th ...
... determined relative to the mass of carbon-12, which by definition is exactly 12 amu. The atomic mass of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. A weighted average mass reflects both the mass and the relative abundance of the isotopes as th ...
AP Chapter 2 Objectives
... established the basic structure of the nuclear atom. 2.3 The Modern View of Atomic Structure I can… ...
... established the basic structure of the nuclear atom. 2.3 The Modern View of Atomic Structure I can… ...
Which has more atoms: a one gram sample of carbon
... Atoms of the same element may have different numbers of neutrons. Carbon may have 6, 7 or 8 neutrons. Hydrogen may have 0, 1 or 2 neutrons. These are called isotopes. Most elements have more than one isotope. Some isotopes are radioactive. Unstable, decay into other elements. Example: ...
... Atoms of the same element may have different numbers of neutrons. Carbon may have 6, 7 or 8 neutrons. Hydrogen may have 0, 1 or 2 neutrons. These are called isotopes. Most elements have more than one isotope. Some isotopes are radioactive. Unstable, decay into other elements. Example: ...
Section 4.2 The Structure of an Atom
... Monitoring Your Understanding Before you read, list in the table shown what you know about atoms and what you would like to learn. After you read, list what you have learned. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the e ...
... Monitoring Your Understanding Before you read, list in the table shown what you know about atoms and what you would like to learn. After you read, list what you have learned. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the e ...
DEMONSTRATE UNDERSTANDING OF ATOMS AND
... few minutes or seconds. Others are very stable and take millions of years to decay to form another atom. The half-life of a radioisotope is the average time it takes for half of the remaining radioactive atoms to decay to a different atom. The radioactivity of any sample will decrease with time as t ...
... few minutes or seconds. Others are very stable and take millions of years to decay to form another atom. The half-life of a radioisotope is the average time it takes for half of the remaining radioactive atoms to decay to a different atom. The radioactivity of any sample will decrease with time as t ...
Chapter 5 The Structure of the Atom
... Students should be able to: • Summarize the essential points of Dalton’s atomic theory. • Describe the particle theory of matter. • Use the Bohr model to differentiate among the three basic particles in an atom • Compare the Bohr atomic model to the electron cloud. ...
... Students should be able to: • Summarize the essential points of Dalton’s atomic theory. • Describe the particle theory of matter. • Use the Bohr model to differentiate among the three basic particles in an atom • Compare the Bohr atomic model to the electron cloud. ...
Chapter 4 Notes
... ____________________ - smallest particle of an element that retains the ____________________ of that element. ____________________ is the man credited with the discovery of the electrons in the late _____, using cathode ray tubes. ____________________ discovered the mass of the electron. Knowledge o ...
... ____________________ - smallest particle of an element that retains the ____________________ of that element. ____________________ is the man credited with the discovery of the electrons in the late _____, using cathode ray tubes. ____________________ discovered the mass of the electron. Knowledge o ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Honors Chem: Atomic History-Isotopes
... Bromine–79 comprises 50.54% of naturally occurring bromine, and Bromine–81 comprises 49.46%. The mass of Br–79 is 78.9183 amu. The mass of Br–81 is 80.9163. What is the atomic mass of bromine? Element X has two naturally occurring isotopes. One isotope has a mass of 35.0 amu and comprises 75.4% by m ...
... Bromine–79 comprises 50.54% of naturally occurring bromine, and Bromine–81 comprises 49.46%. The mass of Br–79 is 78.9183 amu. The mass of Br–81 is 80.9163. What is the atomic mass of bromine? Element X has two naturally occurring isotopes. One isotope has a mass of 35.0 amu and comprises 75.4% by m ...
Element: a pure, simple substance that can`t be broken down into
... What is the smallest unit of matter that we can find everywhere, even in tuna fish? What charge do electrons have? What are elements? Who organized the atomic elements? What do we call a horizontal row on the periodic table? What do we call the vertical columns on the periodic table? The number of p ...
... What is the smallest unit of matter that we can find everywhere, even in tuna fish? What charge do electrons have? What are elements? Who organized the atomic elements? What do we call a horizontal row on the periodic table? What do we call the vertical columns on the periodic table? The number of p ...
T1 Final Study Guide - District 196 e
... *** This study guide is a place to get you started preparing for your final. The final is not limited to only things mentioned on this review, it can be anything we have done over the course of the trimester. We will also be having some review time during class as well. *** ...
... *** This study guide is a place to get you started preparing for your final. The final is not limited to only things mentioned on this review, it can be anything we have done over the course of the trimester. We will also be having some review time during class as well. *** ...
Law of Physics
... • Atomic number determines which element • Mass number determines the isotope-same atomic number, different mass because of amount of neutrons. • There is usually more than one type of isotope for all atoms ...
... • Atomic number determines which element • Mass number determines the isotope-same atomic number, different mass because of amount of neutrons. • There is usually more than one type of isotope for all atoms ...
04 Atom-Review-Worksheet
... a horizontal row of the periodic table stream of electrons produced at the negative electrode of a tube containing a gas at low pressure the central core of an atom, which is composed of protons and neutrons negatively charged subatomic particles subatomic particles with no charge positively charged ...
... a horizontal row of the periodic table stream of electrons produced at the negative electrode of a tube containing a gas at low pressure the central core of an atom, which is composed of protons and neutrons negatively charged subatomic particles subatomic particles with no charge positively charged ...
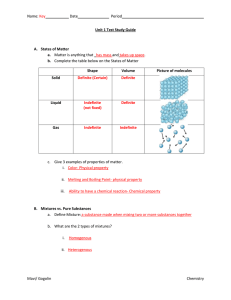
Matter: A) Homogeneous Matter • Uniform and in 1 phase • Even
... Rutherford: Gold foil experiment with alpha particles (positive) bombarding a piece of gold. Most of them went through the foil, but some were deflected. Shows that the atom is mostly empty space and has a small, positive, dense center (nucleus). It disproved Thomson. Also discovered alpha, beta, an ...
... Rutherford: Gold foil experiment with alpha particles (positive) bombarding a piece of gold. Most of them went through the foil, but some were deflected. Shows that the atom is mostly empty space and has a small, positive, dense center (nucleus). It disproved Thomson. Also discovered alpha, beta, an ...
Atomic Structure Scientists
... • Atomos: The point at which matter can no longer be subdivided. ...
... • Atomos: The point at which matter can no longer be subdivided. ...
Chapter 4 and 5 study guide 2016-2017
... Filtering or straining can be used to separate mixtures based on __________________________ ...
... Filtering or straining can be used to separate mixtures based on __________________________ ...
Nuclear Reactions Created by Patrick Haney The atoms of each
... Mass numbers do NOT tell us the number of neutrons in an isotope. To find the number of neutrons in an isotope, you must take the mass number and subtract the atomic number # of neutrons = mass number – atomic number How many neutrons are in the nucleus of a ...
... Mass numbers do NOT tell us the number of neutrons in an isotope. To find the number of neutrons in an isotope, you must take the mass number and subtract the atomic number # of neutrons = mass number – atomic number How many neutrons are in the nucleus of a ...
SCI 3101 Test IV MULTIPLE CHOICE. 1) The sky is blue because air
... 14) If an atom has 43 electrons, 56 neutrons, and 43 protons, what is its approximate atomic mass? What is the name of this element? A) Atomic mass 142 amu; Einsteinium (Es) B) Atomic mass, 99 amu; Radon (Ra) C) Atomic mass, 137 amu; Barium (Ba) D) Atomic mass, 99 amu; Technetium (Tc) 15) Why are t ...
... 14) If an atom has 43 electrons, 56 neutrons, and 43 protons, what is its approximate atomic mass? What is the name of this element? A) Atomic mass 142 amu; Einsteinium (Es) B) Atomic mass, 99 amu; Radon (Ra) C) Atomic mass, 137 amu; Barium (Ba) D) Atomic mass, 99 amu; Technetium (Tc) 15) Why are t ...
Chemistry Midterm Exam 2015 (Study Guide) Unit 1: Measurement
... Convert this temperature –30 C to kelvins. 303K Convert 239 K. to Celsius? -34 What is the formula for density? Mass/vol ...
... Convert this temperature –30 C to kelvins. 303K Convert 239 K. to Celsius? -34 What is the formula for density? Mass/vol ...
electrons
... • Matter was made of tiny particles and could be cut into smaller and smaller pieces until reaching a piece that could not be cut any more. • This smallest piece is an atom. ...
... • Matter was made of tiny particles and could be cut into smaller and smaller pieces until reaching a piece that could not be cut any more. • This smallest piece is an atom. ...
Sub-Atomic Particles and the Nuclear Atom - Chemistry-at-PA
... 57) How many grams of sample are left after 20 minutes if 100 grams start decaying with a half life of 5 minutes? 58) How long will it take 100 grams of radioactive sample to decay so that only 6.25 grams remain and the samples half life is 3 days? 59) How long is the half life of an isotope if 2.0 ...
... 57) How many grams of sample are left after 20 minutes if 100 grams start decaying with a half life of 5 minutes? 58) How long will it take 100 grams of radioactive sample to decay so that only 6.25 grams remain and the samples half life is 3 days? 59) How long is the half life of an isotope if 2.0 ...
Study Guide Answer Key
... Gold Foil Experiment- Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. ...
... Gold Foil Experiment- Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. ...
Atomic Structure Notes Atoms
... Electron cloud or energy rings -Atoms are made of subatomic particles: protons, neutrons, & electrons ...
... Electron cloud or energy rings -Atoms are made of subatomic particles: protons, neutrons, & electrons ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.