REVIEW OF WAVE MECHANICS
... You may be aware of the special role eigenvalue equations play in quantum mechanics. Many of the important equations you have seen have been in the form of Q n q n n , where Q is an operator (e.g. the Hamiltonian on the left hand side of Schrodinger’s equations), and qn and n respectively ar ...
... You may be aware of the special role eigenvalue equations play in quantum mechanics. Many of the important equations you have seen have been in the form of Q n q n n , where Q is an operator (e.g. the Hamiltonian on the left hand side of Schrodinger’s equations), and qn and n respectively ar ...
Formulae, Equations Homework
... into word equations: a) When hydrogen sulphide is burned in oxygen the poisonous gas sulphur dioxide is produced as well as water. b) Iron can be made by heating iron oxide with carbon monoxide in a blast furnace. Carbon dioxide gas is also produced . ...
... into word equations: a) When hydrogen sulphide is burned in oxygen the poisonous gas sulphur dioxide is produced as well as water. b) Iron can be made by heating iron oxide with carbon monoxide in a blast furnace. Carbon dioxide gas is also produced . ...
another Exam2
... Suppose that the first excited state 1 , with wavefunction ! 1 ( x ) = x 1 and energy E1 , is known to be orthogonal to the ground state 0 : 1 0 = 0 . Then let a trial state ! , with wavefunction ! ( x ) = x ! , be some approximation to 1 which is chosen to be orthogonal to 0 : ...
... Suppose that the first excited state 1 , with wavefunction ! 1 ( x ) = x 1 and energy E1 , is known to be orthogonal to the ground state 0 : 1 0 = 0 . Then let a trial state ! , with wavefunction ! ( x ) = x ! , be some approximation to 1 which is chosen to be orthogonal to 0 : ...
optical transitions and excitonic coupling in a covalently linked
... dansyl moiety. Experimentally, a broad fluorescence band with a maximum at 511 nm has been observed [1] which could in principle result as emission from either one of the chromophores or even from both (dual fluorescence). In order to unravel the mechanisms behind the luminescence, we calculate abso ...
... dansyl moiety. Experimentally, a broad fluorescence band with a maximum at 511 nm has been observed [1] which could in principle result as emission from either one of the chromophores or even from both (dual fluorescence). In order to unravel the mechanisms behind the luminescence, we calculate abso ...
CH-103 Tutorial-1
... 3. If the position of speck of dust mass 1 micro gram is known within 10-3 mm, what is the indeterminacy in its momentum and velocity? 4. If an electron in a hydrogen atom is confined to a region of size 53 picometer (pm) from the nucleus, what is the indeterminacy in its momentum and velocity? 5. C ...
... 3. If the position of speck of dust mass 1 micro gram is known within 10-3 mm, what is the indeterminacy in its momentum and velocity? 4. If an electron in a hydrogen atom is confined to a region of size 53 picometer (pm) from the nucleus, what is the indeterminacy in its momentum and velocity? 5. C ...
Variational principle - Indiana University Bloomington
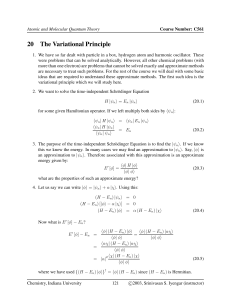
... 1. We have so far dealt with particle in a box, hydrogen atom and harmonic oscillator. These were problems that can be solved analytically. However, all other chemical problems (with more than one electron) are problems that cannot be solved exactly and approximate methods are necessary to treat suc ...
... 1. We have so far dealt with particle in a box, hydrogen atom and harmonic oscillator. These were problems that can be solved analytically. However, all other chemical problems (with more than one electron) are problems that cannot be solved exactly and approximate methods are necessary to treat suc ...
Contents_new - Henry Eyring Center for Theoretical Chemistry
... Session 2: Hartree-Fock: atomic units; electron-nuclear and electron-electron cusps, antisymmetry; Coulomb holes, mean –field potential, Slater determinants, spin-orbitals, spin functions, Slater-Condon rules, The HF equations, Coulomb and exchange, Koopmans’ theorem, orbital energies, problems aris ...
... Session 2: Hartree-Fock: atomic units; electron-nuclear and electron-electron cusps, antisymmetry; Coulomb holes, mean –field potential, Slater determinants, spin-orbitals, spin functions, Slater-Condon rules, The HF equations, Coulomb and exchange, Koopmans’ theorem, orbital energies, problems aris ...
H - unix.eng.ua.edu
... 2. Sij = dij (orthonormal basis set) 3. Hii = a (negative of the ionization potential of the methyl radical) 4. Hij = b (negative stabilization energy). 90º rotation removes all bonding, thus we can calculate DE: DE = 2Ep - Ep where Ep = a and Ep = 2a + 2b (as shown below) ...
... 2. Sij = dij (orthonormal basis set) 3. Hii = a (negative of the ionization potential of the methyl radical) 4. Hij = b (negative stabilization energy). 90º rotation removes all bonding, thus we can calculate DE: DE = 2Ep - Ep where Ep = a and Ep = 2a + 2b (as shown below) ...