D NAME: 1. Which of the following phenomena could not be expla
... wave function that is the superposition of a left-moving particle and a right-moving particle Its energy levels are all negative (g) Its energy levels are not quantized (h) ...
... wave function that is the superposition of a left-moving particle and a right-moving particle Its energy levels are all negative (g) Its energy levels are not quantized (h) ...
Localized Wave Function of the 2D Topological Insulator in a
... honeycomb lattice described by the Kane-Mele (KM) model[1]. It is well know that the KM model with a finite spin-orbit interaction is suggested for a 2D topological insulator[2] which shows an insulating gap in a bulk state and a metallic state on the boundary. This metallic edge state is attributed ...
... honeycomb lattice described by the Kane-Mele (KM) model[1]. It is well know that the KM model with a finite spin-orbit interaction is suggested for a 2D topological insulator[2] which shows an insulating gap in a bulk state and a metallic state on the boundary. This metallic edge state is attributed ...
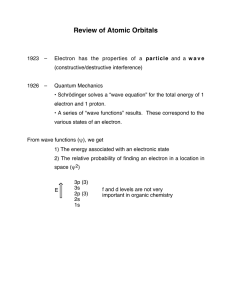
Review of Atomic Orbitals
... • Schrödinger solves a “wave equation” for the total energy of 1 electron and 1 proton. • A series of “wave functions” results. These correspond to the various states of an electron. ...
... • Schrödinger solves a “wave equation” for the total energy of 1 electron and 1 proton. • A series of “wave functions” results. These correspond to the various states of an electron. ...
Honors Chemistry
... 7.4 Determining a compound’s empirical and molecular formula empirical formula - the lowest whole number ratio of atoms in a compound (simplest formula) 3 basic types of problems - first, two rules 1. Divide (%’s or grams) by the gram atomic mass 2. Divide the resulting #’s by the smallest of those ...
... 7.4 Determining a compound’s empirical and molecular formula empirical formula - the lowest whole number ratio of atoms in a compound (simplest formula) 3 basic types of problems - first, two rules 1. Divide (%’s or grams) by the gram atomic mass 2. Divide the resulting #’s by the smallest of those ...
Quantum Mechanical Derivation of the Wallis Formula for $\ pi$
... A famous pre-Newtonian formula for π is obtained directly from the variational approach to the spectrum of the hydrogen atom in spaces of arbitrary dimensions greater than one, including the physical three dimensions. ...
... A famous pre-Newtonian formula for π is obtained directly from the variational approach to the spectrum of the hydrogen atom in spaces of arbitrary dimensions greater than one, including the physical three dimensions. ...
1st Semester Final Exam Review Guide
... Given 238U. Determine the number of protons, neutrons, and electrons. Also determine its mass number. ...
... Given 238U. Determine the number of protons, neutrons, and electrons. Also determine its mass number. ...
Wolfe/Lentz Chapter 6: Chemical Composition Guided Notes 6.1
... 6.2 Atomic Masses: Counting atoms by weighing them To determine the number of molecules, you must know: ...
... 6.2 Atomic Masses: Counting atoms by weighing them To determine the number of molecules, you must know: ...
WODSS SCIENCE SCH 3UI Empirical and Molecular Formula
... Does not tell you __________________________________________________________________. Only tells you the ___________________________________________. Different molecules could have the same percent composition but contain different numbers of atoms in the molecule. Example: Acetylene and Benzene Pro ...
... Does not tell you __________________________________________________________________. Only tells you the ___________________________________________. Different molecules could have the same percent composition but contain different numbers of atoms in the molecule. Example: Acetylene and Benzene Pro ...
representing chemical compounds
... Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 8. The molecular formula for table salt is NaCl. ________ 9. Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other eleme ...
... Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 8. The molecular formula for table salt is NaCl. ________ 9. Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other eleme ...
Systematic improvement of the correlation energy of solids
... ACFDT. Among the possible ways to proceed, the accuracy of the response functions used within the ACFDT framework will be improved by applying timedependent density functional theory (TDDFT). Different approximations of the TDDFT kernel will be tested to understand their influence on the accuracy of ...
... ACFDT. Among the possible ways to proceed, the accuracy of the response functions used within the ACFDT framework will be improved by applying timedependent density functional theory (TDDFT). Different approximations of the TDDFT kernel will be tested to understand their influence on the accuracy of ...



![3 — Blackbody Radiation [Revision : 1.5]](http://s1.studyres.com/store/data/005908504_1-5005bdffc2e5f9c6e0687c31f49c7e9d-300x300.png)