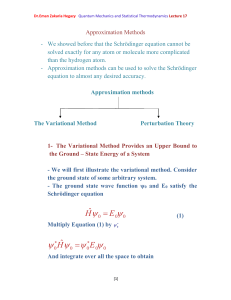
Breaking Multiple Covalent Bonds with Hartree-Fock-based
... method for the calculation of ground-state molecular electronic structure by augmenting it with the standard perturbative (T) correction for the effects of connected triple excitations. We demonstrate the OQVCCD(T) ansatz to be outstandingly robust and accurate in the description of the breaking of ...
... method for the calculation of ground-state molecular electronic structure by augmenting it with the standard perturbative (T) correction for the effects of connected triple excitations. We demonstrate the OQVCCD(T) ansatz to be outstandingly robust and accurate in the description of the breaking of ...
Chapter 2 Part 1 ppt
... • When energy is absorbed, electrons move to higher orbits • When electrons move to lower orbits, energy is emitted • Equation predicted line spectra only for single-electron atoms • Adjustments were made to use elliptical orbits to better fit data • Ultimately failed - did not incorporate wave prop ...
... • When energy is absorbed, electrons move to higher orbits • When electrons move to lower orbits, energy is emitted • Equation predicted line spectra only for single-electron atoms • Adjustments were made to use elliptical orbits to better fit data • Ultimately failed - did not incorporate wave prop ...
2/25/11 QUANTUM MECHANICS II (524) PROBLEM SET 6 (hand in
... electron). The electron angular momentum is denoted by J = L + S, where L is the orbital angular momentum of the electron and S its spin. The total angular momentum of the atom is F = J + I, where I is the nuclear spin. a) What are the possible values of the quantum numbers J and F for a deuterium a ...
... electron). The electron angular momentum is denoted by J = L + S, where L is the orbital angular momentum of the electron and S its spin. The total angular momentum of the atom is F = J + I, where I is the nuclear spin. a) What are the possible values of the quantum numbers J and F for a deuterium a ...
Quantum Mechanics Lecture Course for 4 Semester Students by W.B. von Schlippe
... relationship between the concepts of frequency and energy, we start in this paper from the existence of a certain periodic process of an as yet not more closely specified nature which must be assigned to each isolated portion of energy and which depends on its eigenmass in accordance with the Planck ...
... relationship between the concepts of frequency and energy, we start in this paper from the existence of a certain periodic process of an as yet not more closely specified nature which must be assigned to each isolated portion of energy and which depends on its eigenmass in accordance with the Planck ...
Quantum numbers
... When the electron density map is divided into equal spheres, the plot of finding the electron in each successive sphere gives the following curve ...
... When the electron density map is divided into equal spheres, the plot of finding the electron in each successive sphere gives the following curve ...