An Overview of Computational Chemistry
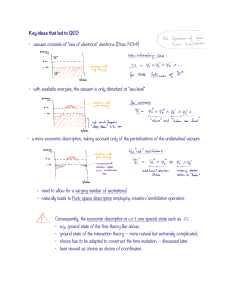
... •The BO approx. is usually very good, but breaks down when two (or more) electronic states are close in energy at particular nuclear geometries. •In such situations, a “ non-adiabatic” wave function - a product of nuclear and electronic wave functions - must be used. The electronic Hamiltonian becom ...
... •The BO approx. is usually very good, but breaks down when two (or more) electronic states are close in energy at particular nuclear geometries. •In such situations, a “ non-adiabatic” wave function - a product of nuclear and electronic wave functions - must be used. The electronic Hamiltonian becom ...
Localization of the eigenfunctions and associated free boundary problems
... The phenomenon of wave localization permeates acoustics, quantum physics, energy engineering. It was used in the construction of noise abatement walls, LEDs, optical devices. Localization of quantum states of electrons by a disordered potential has become one of the prominent subjects in quantum phy ...
... The phenomenon of wave localization permeates acoustics, quantum physics, energy engineering. It was used in the construction of noise abatement walls, LEDs, optical devices. Localization of quantum states of electrons by a disordered potential has become one of the prominent subjects in quantum phy ...
Click here - Louisiana State University
... bulk state, gold clusters with a core diameter less than 2 nm are characterized by distinct quantum confinement effects. As a result discrete electronic structure and molecular type properties such as HUMO-LUMO transitions and intrinsic magnetism are observed. A major challenge in gold cluster synth ...
... bulk state, gold clusters with a core diameter less than 2 nm are characterized by distinct quantum confinement effects. As a result discrete electronic structure and molecular type properties such as HUMO-LUMO transitions and intrinsic magnetism are observed. A major challenge in gold cluster synth ...
De Broglie Waves.
... Solution of Schrodinger equation (SWE) The solution of the equation means that we must know the Hamiltonian operator to get the wave function and total energy The wave functions contain all the dynamical information about systems they describe (position, momentum, angular momentum, energy) T ...
... Solution of Schrodinger equation (SWE) The solution of the equation means that we must know the Hamiltonian operator to get the wave function and total energy The wave functions contain all the dynamical information about systems they describe (position, momentum, angular momentum, energy) T ...