Tutorial 1 - NUS Physics
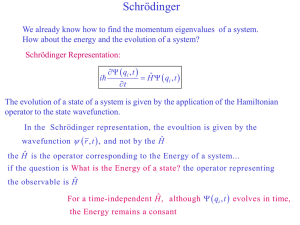
... In the abstract Hilbert space H, the energy eigenvectors of a particle in an infinite square well of width L are designated by E1 , E2 , E3 ,, for the ground state, the first excited state, the second excited state, and so on. Suppose that at a given time a particle is in the state ...
... In the abstract Hilbert space H, the energy eigenvectors of a particle in an infinite square well of width L are designated by E1 , E2 , E3 ,, for the ground state, the first excited state, the second excited state, and so on. Suppose that at a given time a particle is in the state ...
6. Quantum Mechanics II
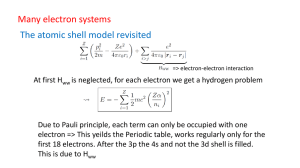
... Quantum mechanics applied to the Hydrogen atom: quantum number, energy and angular momentum Study Chapters 5 and 7 hard! ...
... Quantum mechanics applied to the Hydrogen atom: quantum number, energy and angular momentum Study Chapters 5 and 7 hard! ...
Guidelines for Abstract
... 3. Discussion We report the detailed theoretical analysis of the properties of the effective potential associated with an exact wave function. Furthermore, as an elementary application of the present formalism, we propose a direct method to calculate the so-called Brueckner orbitals [7] as a special ...
... 3. Discussion We report the detailed theoretical analysis of the properties of the effective potential associated with an exact wave function. Furthermore, as an elementary application of the present formalism, we propose a direct method to calculate the so-called Brueckner orbitals [7] as a special ...