The Schrödinger equation
... 1. The TDSE is one of the postulates of quantum mechanics. Though the SE cannot be derived, it has been shown to be consistent with all experiments. 2. SE is first order with respect to time (cf. classical wave equation). 3. SE involves the complex number i and so its solutions are essentially compl ...
... 1. The TDSE is one of the postulates of quantum mechanics. Though the SE cannot be derived, it has been shown to be consistent with all experiments. 2. SE is first order with respect to time (cf. classical wave equation). 3. SE involves the complex number i and so its solutions are essentially compl ...
Lecture 12
... lower energy. It is also called an annihilation operator, because it removes one quantum of energy �ω from the system. Similarly it is straightforward to show that Ĥ↠|n� = (En + �ω)↠|n� , which says that ↠|n� is an eigenfunction of Ĥ belonging to the eigenvalue (En + �ω), unless ↠|n� ≡ ...
... lower energy. It is also called an annihilation operator, because it removes one quantum of energy �ω from the system. Similarly it is straightforward to show that Ĥ↠|n� = (En + �ω)↠|n� , which says that ↠|n� is an eigenfunction of Ĥ belonging to the eigenvalue (En + �ω), unless ↠|n� ≡ ...
Lecture 4
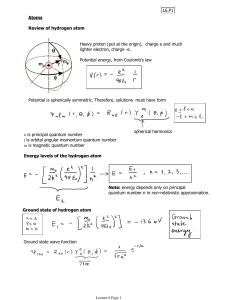
... 2. Is it degenerate? 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
... 2. Is it degenerate? 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
x - Purdue Physics
... “down the drain” into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. - Richard Feynman Those who are not shocked when they first come across quantum mechanics cannot possibly have understood it. Richard Feynman (1918-1988) ...
... “down the drain” into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. - Richard Feynman Those who are not shocked when they first come across quantum mechanics cannot possibly have understood it. Richard Feynman (1918-1988) ...
Empirical Formula
... • A counting number (like a dozen) • 6.02 X 1023 (in scientific notation) • This number is named in honor ...
... • A counting number (like a dozen) • 6.02 X 1023 (in scientific notation) • This number is named in honor ...