* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Click here to Ch 07.4 Determining Chemical Formulas
Survey
Document related concepts
Transcript
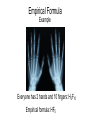
Ch. 7.4 Determining Chemical Formulas Ch. 7.4 Determining Chemical Formulas Objectives • Define empirical formula, and explain how the term applies to ionic and molecular compounds. • Determine an empirical formula from either a percentage or a mass composition. • Explain the relationship between the empirical formula and the molecular formula of a given compound. • Determine a molecular formula from an empirical formula. Ch. 7.4 Determining Chemical Formulas • An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratio of the different atoms in the compound. • For an ionic compound, the formula unit is usually the compound’s empirical formula. • For a molecular compound, however, the empirical formula does not necessarily indicate the actual numbers of atoms present in each molecule. Empirical Formula Example Everyone has 2 hands and 10 fingers: H2F10 Empirical formula: HF5 Empirical Formula Benzene Molecular Formula: C6H6 Proportion of C to H is 1:1 Empirical Formula: CH More than one substance may reduce to the same empirical formula. Benzene: C6H6 Acetylene: C2H2 Ch. 7.4 Determining Chemical Formulas Calculation of Empirical Formulas • To determine a compound’s empirical formula from its percentage composition, begin by converting percentage composition to a mass composition. • Assume that you have a 100.0 g sample of the compound. • Then calculate the amount of moles of each element in the sample. • Simplest ratio of moles Ch. 7.4 Determining Chemical Formulas Empirical Formula We have a sample that is 48.6% C, 8.16% H, and 43.2% O by weight. What is the empirical formula? Step 1: % Composition to Moles 48.6% C = 48.6g C; 8.16% H = 8.16g H; 43.2% O = 43.2g O; 48.6g / 12.01g/mol 8.16g / 1.01g/mol 43.2g / 16.00g/mol = 4.05 mol C = 8.08 mol H = 2.70 mol O Ch. 7.4 Determining Chemical Formulas Empirical Formula Step 2: Mole Ratio Divide each element by the smallest number of moles C: 4.05 mol / 2.70 mol = 1.5 H: 8.08 mol / 2.70 mol = 2.99 RU = 3 O: 2.70 mol / 2.70 mol = 1 Ch. 7.4 Determining Chemical Formulas Empirical Formula Step 3: Atom Ratio Ratio of atoms MUST be a whole number! C = 1.5 x 2 = 3 H=3x2=6 O=1x2=2 Ch. 7.4 Determining Chemical Formulas Empirical Formula Step 4: Express as an Empirical Formula C 3H 6 O 2 Ch. 7.4 Determining Chemical Formulas Molecular Formula Given the composition percentages and the samples Molecular Mass. First: We use the percent composition to Empirical Formula Rules. Weight % → Mass → Mol → Ratio of Mol → Atoms = Empirical Formula Ch. 7.4 Determining Chemical Formulas Molecular Formula Second: Calculate the Empirical Mass from the Empirical Formula. Third: Divide the Molecular Mass (given) by Empirical Mass (MM/ EM) = n Last: Multiply Empirical Formula subscripts by n (EF)n = Molecular Formula Ch. 7.4 Determining Chemical Formulas Molecular Formula A compound is 92.30% C and 7.80% H with a mass of 78.12g/mol. Find the empirical formula and the molecular formula of this compound. Ch. 7.4 Determining Chemical Formulas Molecular Formula Mass to mol: C: 92.30g/ 12.01 = 7.69 mol H: 7.80g/ 1.01 = 7.72 mol Ratio of Mol: C: 7.69 / 7.69 = 1 H: 7.72/ 7.69 = 1 Atoms: C:H 1:1 Empirical Formula (EF): CH Ch. 7.4 Determining Chemical Formulas Molecular Formula Empirical Mass (EM): (12.01) + (1.01)= 13.02 n = (MM/EM): 78.12 / 13.02 = 6 (EF)n: (CH) (6)= C6H6 Ch. 7.4 Determining Chemical Formulas Molecular Formula Sample Problem The empirical formula of a compound of phosphorus and oxygen was found to be P2O5. Experimentation shows that the molar mass of this compound is 283.89 g/mol. What is the compound’s molecular formula?