Chemistry 101 2007
... Most elements can interact with other elements to form compounds (which cannot?). e.g. H2 and O2 combine with each other to form water, H2O. The properties of water are very different from those of H2 and O2. ...
... Most elements can interact with other elements to form compounds (which cannot?). e.g. H2 and O2 combine with each other to form water, H2O. The properties of water are very different from those of H2 and O2. ...
File
... D) An observation explains why nature does something. E) A scientific law summarizes a series of related observations 3) Which of the following statements about the phases of matter is TRUE? A) In both solids and liquids, the atoms or molecules pack closely to one another. B) Solids are highly compr ...
... D) An observation explains why nature does something. E) A scientific law summarizes a series of related observations 3) Which of the following statements about the phases of matter is TRUE? A) In both solids and liquids, the atoms or molecules pack closely to one another. B) Solids are highly compr ...
PHYSICAL CHEMISTRY ERT 108 Semester II 2010
... is the partial pressure of gas i in the mixture, and is the chemical potential of pure ideal gas i at the standard pressure of 1 bar and at the same temperature T as the mixture. ...
... is the partial pressure of gas i in the mixture, and is the chemical potential of pure ideal gas i at the standard pressure of 1 bar and at the same temperature T as the mixture. ...
midterm Practice examination answer Key
... Blank” questions. As each blank is worth one mark, some questions will have a total value of two marks. Note that there are MORE terms provided than you need, so read over the list carefully and choose the terms you want to use. The same term may be used more than once in this section. Physical Prop ...
... Blank” questions. As each blank is worth one mark, some questions will have a total value of two marks. Note that there are MORE terms provided than you need, so read over the list carefully and choose the terms you want to use. The same term may be used more than once in this section. Physical Prop ...
Unit 1 - Learning Objectives
... (iii) Isotopes Isotopes are atoms with the same atomic number but different mass numbers. Most elements exist as a mixture of isotopes. The relative atomic mass of an element is rarely a whole number. d) Bonding, structure and properties Bonding Atoms can be held together by bonds. In form ...
... (iii) Isotopes Isotopes are atoms with the same atomic number but different mass numbers. Most elements exist as a mixture of isotopes. The relative atomic mass of an element is rarely a whole number. d) Bonding, structure and properties Bonding Atoms can be held together by bonds. In form ...
Describing Matter
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
In chemistry the ideal gas law combines Boyle`s Law, which relates
... where p is the pressure exerted by the gas in a closed container, V is the volume of the container, n is the number of moles of the gas present in a closed container, R* is the universal gas constant, and T is the absolute temperature of the gas. (R* is essentially a constant of proportionality betw ...
... where p is the pressure exerted by the gas in a closed container, V is the volume of the container, n is the number of moles of the gas present in a closed container, R* is the universal gas constant, and T is the absolute temperature of the gas. (R* is essentially a constant of proportionality betw ...
Review Session Handout from 10/6
... 1. How many moles of solute are present in the following solutions: (a) 65.0 mL of 0.125 M HNO3? (b) 120.0 mL of 1.500 M NaCl? 2. How many grams of solute are required to prepare the following solutions: (a) 100.0 mL of 0.400 M H3BO3? (b) 500.0 mL of 1.20 M HCl? 3. How many moles of Cl- ions are pre ...
... 1. How many moles of solute are present in the following solutions: (a) 65.0 mL of 0.125 M HNO3? (b) 120.0 mL of 1.500 M NaCl? 2. How many grams of solute are required to prepare the following solutions: (a) 100.0 mL of 0.400 M H3BO3? (b) 500.0 mL of 1.20 M HCl? 3. How many moles of Cl- ions are pre ...
Pressure Units
... In metric units, pressure if Newtons (force) per square meter (area). One Newton is not very much pressure… about the weight of a small apple (get it… apple… Newton)… and if that force is exerted over a square meter, the amount of pressure is very small and called a pascal (Pa). It is more useful to ...
... In metric units, pressure if Newtons (force) per square meter (area). One Newton is not very much pressure… about the weight of a small apple (get it… apple… Newton)… and if that force is exerted over a square meter, the amount of pressure is very small and called a pascal (Pa). It is more useful to ...
Lecture notes 11
... and ionization state because free electrons must be counted. Complete treatment needs to use Saha Equation to account for everything. We will consider two cases (1) gas all neutral and (2) gas all ionized. ...
... and ionization state because free electrons must be counted. Complete treatment needs to use Saha Equation to account for everything. We will consider two cases (1) gas all neutral and (2) gas all ionized. ...
the properties and structure of matter
... be observed or measured without changing the identity or composition of the substance • Physical properties used to describe matter can be classified as: 1) Extensive – depends on the amount of matter in the sample - e.g. Mass, volume, length 2) Intensive – depends on the type of matter, not the amo ...
... be observed or measured without changing the identity or composition of the substance • Physical properties used to describe matter can be classified as: 1) Extensive – depends on the amount of matter in the sample - e.g. Mass, volume, length 2) Intensive – depends on the type of matter, not the amo ...
INTRODUCTION TO CHEMISTRY
... __PHYSICAL__ properties can be observed without chemically changing matter. ___CHEMICAL_____ properties describe how a substance interacts with other substances. __SOLIDS___ have definite shapes and definite volumes. __LIQUIDS_____ have indefinite shapes and definite volumes. ___GASES/ PLASMA______ ...
... __PHYSICAL__ properties can be observed without chemically changing matter. ___CHEMICAL_____ properties describe how a substance interacts with other substances. __SOLIDS___ have definite shapes and definite volumes. __LIQUIDS_____ have indefinite shapes and definite volumes. ___GASES/ PLASMA______ ...
CHEM 101 Final (Term 141)
... substance B are lower than those for substance A. B) The pressure at the triple point, normal boiling and normal melting point for substance A are lower than those of substance B. C) The pressure at the triple point for substance A is higher than that of substance B, but the normal boiling and norma ...
... substance B are lower than those for substance A. B) The pressure at the triple point, normal boiling and normal melting point for substance A are lower than those of substance B. C) The pressure at the triple point for substance A is higher than that of substance B, but the normal boiling and norma ...
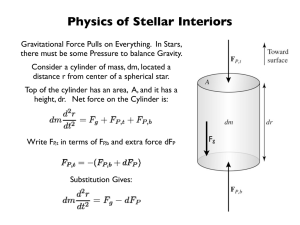
Ch12&13 Life and Death of Stars
... • Adding mass to a white dwarf increases its gravity, forcing electrons into a smaller space • In order to avoid being in the same state some of the electrons need to move faster ...
... • Adding mass to a white dwarf increases its gravity, forcing electrons into a smaller space • In order to avoid being in the same state some of the electrons need to move faster ...
Chapter 9: Chemical Bonding I: Lewis Theory
... 1) Ionic Bonding A) Ionic Bonding results from electron transfer. B) Occurs between metals & nonmetals. i) Metals lose electrons to form cations while nonmetals gain electrons to form anions. C) Ion pair is more stable than separated ions. D) Found as a 3-D crystal lattices containing alternating ca ...
... 1) Ionic Bonding A) Ionic Bonding results from electron transfer. B) Occurs between metals & nonmetals. i) Metals lose electrons to form cations while nonmetals gain electrons to form anions. C) Ion pair is more stable than separated ions. D) Found as a 3-D crystal lattices containing alternating ca ...
Chapter One Chemistry
... isproperty made atoms that of two is combine has a or characteristic mass more toand form substances— takes larger of aup pure particles space. substance called elements, molecules—groups compounds, that describes or its both—that ofability two or tomore are change together atoms into held in Chemis ...
... isproperty made atoms that of two is combine has a or characteristic mass more toand form substances— takes larger of aup pure particles space. substance called elements, molecules—groups compounds, that describes or its both—that ofability two or tomore are change together atoms into held in Chemis ...
ME 215
... Sometimes the density of a substance is given relative to the density of a wellknown substance. Then it is called specific gravity, or relative density, and is defined as the ratio of the density of a substance to the density of some standard substance at a specified ...
... Sometimes the density of a substance is given relative to the density of a wellknown substance. Then it is called specific gravity, or relative density, and is defined as the ratio of the density of a substance to the density of some standard substance at a specified ...
CHEM 230: Principles of Physical Chemistry
... tend to reduce the pressure. • The term nb accounts for the small but finite volume occupied by the gas molecules. • The equation adjusts the volume downward by subtracting nb to give the volume that would be available to the molecules in the ideal case. WATCH THE UNITS OF “a” and “b” constants! ...
... tend to reduce the pressure. • The term nb accounts for the small but finite volume occupied by the gas molecules. • The equation adjusts the volume downward by subtracting nb to give the volume that would be available to the molecules in the ideal case. WATCH THE UNITS OF “a” and “b” constants! ...
Basic Chemistry Lecture Notes - Roderick Biology
... • Electrons can be found on electron shells • Electrons on the outermost electron shell are called valence electrons Valence Electron e- ...
... • Electrons can be found on electron shells • Electrons on the outermost electron shell are called valence electrons Valence Electron e- ...
5-th_state_matter - The 5th state of matter
... Summary: beautiful filament-systems are often shown by the astonishing development of the modern astronomy. Most of these filaments have an exact circular cross section. Filaments have the same interesting characteristics from a diameter of 0.01 mm to that of many 1000 of light-years. Filaments are ...
... Summary: beautiful filament-systems are often shown by the astonishing development of the modern astronomy. Most of these filaments have an exact circular cross section. Filaments have the same interesting characteristics from a diameter of 0.01 mm to that of many 1000 of light-years. Filaments are ...
- The 5th state of matter
... t e astonishing development of the modern astronomy. Most of these filaments have an exact circular cross section. Filaments have the same interesting characteristics from a diameter of 0.01 mm to that of many 1000 of light-years. Filaments are incorrectly seen to be of plasma, however, particles mo ...
... t e astonishing development of the modern astronomy. Most of these filaments have an exact circular cross section. Filaments have the same interesting characteristics from a diameter of 0.01 mm to that of many 1000 of light-years. Filaments are incorrectly seen to be of plasma, however, particles mo ...
Vapor Pressure of a Pure Liquid
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...