* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 9: Chemical Bonding I: Lewis Theory
Surface properties of transition metal oxides wikipedia , lookup
X-ray fluorescence wikipedia , lookup
State of matter wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Electrochemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Molecular orbital wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Electron scattering wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Heat transfer physics wikipedia , lookup
Degenerate matter wikipedia , lookup
Photoelectric effect wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Aromaticity wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
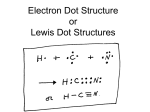
69 Chapter 9. Chemical Bonding I: Lewis Theory I) Types of Bonding: General Concepts 1) Ionic Bonding A) Ionic Bonding results from electron transfer. B) Occurs between metals & nonmetals. i) Metals lose electrons to form cations while nonmetals gain electrons to form anions. C) Ion pair is more stable than separated ions. D) Found as a 3-D crystal lattices containing alternating cations & anions. 2) Covalent Bonding A) Covalent Bonding results from sharing valence electrons. B) Occurs between nonmetals. C) Most important kind of bond in chemistry. 3) Metallic Bonding a) Occurs in metals. b) Electron Sea Model: we will not focus on this at all. II) Nature & Strength of Covalent Bonds 1) Associated terminology A) Bond Energy - energy required to break a bond (also called bond dissociation energy). B) Bond Length - distance between atoms where energy is minimized. - As energy of system is minimized, its stability increases. 2) Types of Covalent Bonds A) Polar Covalent Bonding [ H2O, NH3, HF ] - unequal sharing of valence electrons, but not to the point of being electron transfer (ionic bonding). - characterized by formation of a dipole. HF + - 70 B) Nonpolar Covalent Bonding [ CO, CH4 ] - equal sharing of valence electrons, no dipole exists. - found with symmetrical molecules (dipoles all cancel out). III) Comparing Ionic & Covalent Compounds 1) Refer to Table 9.1 & be familiar with general trends. IV) Polar Covalent Bonds: Electronegativity [Figure 9.10 & 9.11] 1) Electronegativity refers to the ability of atom to attract shared electrons to itself. 2) Symbolized by Greek symbol A) Electronegativity scale developed by Pauling [ F = 4.0 ] B) between two atoms = measure of ionic character. 3) increases across period and decreases going down a column. V) Lewis Dot Structures 1) Shorthand notation denoting the valence electrons around an atom. 2) Valence electrons can be looked upon in two ways. A) bonding pair electrons - electrons found in space between atoms. B) lone pair (nonbonding) electrons - electrons found around an atom. 3) Electrons are represented as dashes (bonding electrons) and as dots (lone pair or nonbonding electrons). 4) Chemical Bonds A) Types of Bonds i. single bonds - 1 pair of shared valence electrons. ii. double bonds - 2 pairs of shared valence electrons. iii. triple bonds - 3 pairs shared valence electrons. C-C C=C CC B) As the number of bonds increases, the bond energy increases and the bond length decreases. 71 VI) Drawing Lewis Structures 1) See Handout for How to Draw Lewis Structures. 2) General Principles to Remember A) Hydrogen 2 electrons (max.) B) Octet Rule 8 electrons (max.) C) 18 Electron Rule duet rule 2s & 2p elements n = 3 or beyond i) one of three classes of exceptions to octet rule (see handout). ii) break octet rule due to available empty d-orbitals 3) Examples A) carbon dioxide .. .. :O=C=O: B) cyanide ion [ :C N: ]- 4) Resonance A) Resonance occurs when more than 1 Lewis structure can be drawn for a molecule. Resonance forms only differ in the placement of valence electrons. i) ii) iii) The total number of valence electrons remains the same in all resonance structures. The placement of atoms remains the same. The connectivity of the atoms remains the same. B) Actual structure of molecule is represented by the average of all possible Lewis structures. C) Lewis structures are denoted by a double arrow ( ) D) Examples - nitrate ion ( 3 possible Lewis Structures) 5) Formal Charge A) Another kind of Electron Bookkeeping. B) Difference between the number of valence electrons on the free atom and the number of valence electrons assigned to the atom in the molecule. 72 Formal Charge = Group # - # bonds - # lone electrons C) Best Lewis Structure for a Molecule is one in which i) formal charge separation is minimized throughout the molecule ii) any negative formal charges are found on the more electronegative element (e.g., O, N, etc.). D) Example - nitrate ion & sulfate ion