Final Analysis – Exam Review
... a. An electrical device that impedes the flow of electrons b. A measure of the amount of energy that each electron possesses c. The number of electrons that pass through the wire in a specific time interval d. Measured in a circuit using a voltmeter 2. Electric potential (Voltage) is: a. The number ...
... a. An electrical device that impedes the flow of electrons b. A measure of the amount of energy that each electron possesses c. The number of electrons that pass through the wire in a specific time interval d. Measured in a circuit using a voltmeter 2. Electric potential (Voltage) is: a. The number ...
Unit Conversion for Gas Laws Calculations
... The particles are assumed to exert no forces on each other; they are assumed to neither attract nor repel each other. The average kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas. ...
... The particles are assumed to exert no forces on each other; they are assumed to neither attract nor repel each other. The average kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas. ...
compounds - Net Start Class
... 22. Which of the following is NOT and example of a CHEMICAL CHANGE? a. Compounds may be broken down into elements. b. Elements may combine to form compounds. c. Compounds may change from one state of matter to another. d. Compounds may change into other compounds. ...
... 22. Which of the following is NOT and example of a CHEMICAL CHANGE? a. Compounds may be broken down into elements. b. Elements may combine to form compounds. c. Compounds may change from one state of matter to another. d. Compounds may change into other compounds. ...
File
... shells, either by gaining or losing electrons (ionic bonds), or by sharing electrons (covalent bonds). ...
... shells, either by gaining or losing electrons (ionic bonds), or by sharing electrons (covalent bonds). ...
Chapter 12 Star Stuff How do stars form?
... • Degeneracy Pressure comes from the quantum nature of matter • Two quantum particles—electrons, nuclei, etc—cannot be in the same place at the same time • So they can only get so close • When a protostar has contracted to the point where it can’t contract anymore without particles being in the same ...
... • Degeneracy Pressure comes from the quantum nature of matter • Two quantum particles—electrons, nuclei, etc—cannot be in the same place at the same time • So they can only get so close • When a protostar has contracted to the point where it can’t contract anymore without particles being in the same ...
Chemical Context of Life
... • there is a natural tendency for matter to move to the lowest state • potential energy of electrons is not infinitely divisible, but exists only in discrete amounts called quanta • different fixed potential energy states for electrons are called levels or electron shells: • electrons with lowest po ...
... • there is a natural tendency for matter to move to the lowest state • potential energy of electrons is not infinitely divisible, but exists only in discrete amounts called quanta • different fixed potential energy states for electrons are called levels or electron shells: • electrons with lowest po ...
Vocabulary Notes
... A mixture of two or more metals or metals and non-metals that result in the mixture having different properties than their component elements. Ex. Stainless Steel. ...
... A mixture of two or more metals or metals and non-metals that result in the mixture having different properties than their component elements. Ex. Stainless Steel. ...
Document
... chlorofluorocarbons (CFCs), nitrogen oxides found in smog, and volatile organic compounds (VOCs), such as compounds found in paint thinners. ...
... chlorofluorocarbons (CFCs), nitrogen oxides found in smog, and volatile organic compounds (VOCs), such as compounds found in paint thinners. ...
Chem 2 AP Ch 7 MC Review Key
... 18. Which electronic transition requires the addition of the most energy? [The only one where ni < nf] A) n=1 to n=3 D) n=4 to n=1 B) n=5 to n=2 E) n=5 to n=1 C) n=2 to n=3 19. Electromagnetic radiation behaves like particles when it A) travels through space B) is absorbed by matter C) interacts wit ...
... 18. Which electronic transition requires the addition of the most energy? [The only one where ni < nf] A) n=1 to n=3 D) n=4 to n=1 B) n=5 to n=2 E) n=5 to n=1 C) n=2 to n=3 19. Electromagnetic radiation behaves like particles when it A) travels through space B) is absorbed by matter C) interacts wit ...
Lecture7
... eventually end up as white dwarfs. However, before the degeneracy sets in finally in the core, elements heavier than C and O would be synthesized. So, their core will consist of elements heavier than C and O, e.g., Mg, Si, but not Fe. However, some calculations show that some of these stars on the h ...
... eventually end up as white dwarfs. However, before the degeneracy sets in finally in the core, elements heavier than C and O would be synthesized. So, their core will consist of elements heavier than C and O, e.g., Mg, Si, but not Fe. However, some calculations show that some of these stars on the h ...
ap unit 5 worksheet answers
... 29. Under what conditions of temp. and pressure do gases usually behave nonideally? Low temp and high pressure 30. Would you expect water or carbon dioxide to behave more like an ideal gas at high pressures? Carbon dioxide 31. How do viscosity and surface tension change as intermolecular forces beco ...
... 29. Under what conditions of temp. and pressure do gases usually behave nonideally? Low temp and high pressure 30. Would you expect water or carbon dioxide to behave more like an ideal gas at high pressures? Carbon dioxide 31. How do viscosity and surface tension change as intermolecular forces beco ...
ก F ก F U234 92
... are the partial pressure of these substances in the vapor above a solution of 1.60 mol of CH2Cl2 and 1.10 mol of CCl4 at 23.5ºC? The vapor pressures of pure CH2Cl2 and CCl4 at 23.5ºC are 352 torr and 118 torr, respectively. (Assume ideal behavior.) 6. Sketch a qualitative enthalpy diagram for the pr ...
... are the partial pressure of these substances in the vapor above a solution of 1.60 mol of CH2Cl2 and 1.10 mol of CCl4 at 23.5ºC? The vapor pressures of pure CH2Cl2 and CCl4 at 23.5ºC are 352 torr and 118 torr, respectively. (Assume ideal behavior.) 6. Sketch a qualitative enthalpy diagram for the pr ...
1.3 Accretion power in astrophysics
... object in a binary system: a) standard collapse mechanism for formation of neutron star/bh, provided that the binary system was able to survive the supernova explosion}. In the case of the high-mass X-ray binaries, this survival is a clear consequence of the large-scale mass transfer which precedes ...
... object in a binary system: a) standard collapse mechanism for formation of neutron star/bh, provided that the binary system was able to survive the supernova explosion}. In the case of the high-mass X-ray binaries, this survival is a clear consequence of the large-scale mass transfer which precedes ...
Real Gases
... and so will not "condense" into a liquid. It is already extremely dense and behaving somewhat as a liquid already. The density of a supercritical fluid is more like that of a liquid than a gas, but is significantly less than for the liquid at ordinary conditions. For example, … the density of H 2O a ...
... and so will not "condense" into a liquid. It is already extremely dense and behaving somewhat as a liquid already. The density of a supercritical fluid is more like that of a liquid than a gas, but is significantly less than for the liquid at ordinary conditions. For example, … the density of H 2O a ...
Chapter 7:The Quantum-Mechanical Model of
... Dalton’s Indivisible Atom explained the Law of Constant Composition and the Law of Conservation of Mass and led to the Law of Multiple Proportions. J.J. Thomson, through Cathode Ray Tube experiments, discovered that electrons are small negatively charged particles inside a divisible atom and came up ...
... Dalton’s Indivisible Atom explained the Law of Constant Composition and the Law of Conservation of Mass and led to the Law of Multiple Proportions. J.J. Thomson, through Cathode Ray Tube experiments, discovered that electrons are small negatively charged particles inside a divisible atom and came up ...
Chemistry Review
... Fluids – gases and liquids, flow Ideal gas – imaginary gas that fits all the assumptions of the kinetic molecular theory Kelvin – SI unit of temperature Kinetic Theory- group of ideas explaining the interaction of matter and energy due to particle motion Melting – change in state from a solid to a l ...
... Fluids – gases and liquids, flow Ideal gas – imaginary gas that fits all the assumptions of the kinetic molecular theory Kelvin – SI unit of temperature Kinetic Theory- group of ideas explaining the interaction of matter and energy due to particle motion Melting – change in state from a solid to a l ...
Real Gases
... @ T = 200 K, z initially decreases with pressure. The compression factor only becomes greater than the ideal gas value of one for pressures in excess of 200 bar. For T = 400. K, z increases linearly with T. This functional dependence is predicted by the Redlich-Kwong equation. The van der Waals equa ...
... @ T = 200 K, z initially decreases with pressure. The compression factor only becomes greater than the ideal gas value of one for pressures in excess of 200 bar. For T = 400. K, z increases linearly with T. This functional dependence is predicted by the Redlich-Kwong equation. The van der Waals equa ...
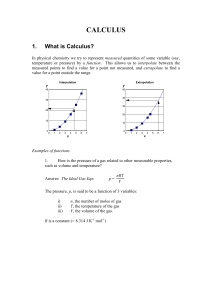
CALCULUS
... 3. How much heat energy must we provide to raise the temperature of a liquid from 0C to 100C, given that its heat capacity is a function of temperature? Answer: The amount of heat energy needed is given by the area under the heat capacity vs. T graph (you’ll learn this in 2nd year thermodynamics). ...
... 3. How much heat energy must we provide to raise the temperature of a liquid from 0C to 100C, given that its heat capacity is a function of temperature? Answer: The amount of heat energy needed is given by the area under the heat capacity vs. T graph (you’ll learn this in 2nd year thermodynamics). ...
Export To Word
... change in motion. When objects travel at speeds comparable to the speed of light, Einstein's special theory of relativity applies. B. Momentum is conserved under well-defined conditions. A change in momentum occurs when a net force is applied to an object over a time interval. C. The Law of Universa ...
... change in motion. When objects travel at speeds comparable to the speed of light, Einstein's special theory of relativity applies. B. Momentum is conserved under well-defined conditions. A change in momentum occurs when a net force is applied to an object over a time interval. C. The Law of Universa ...
Condensates in Neutron Star Interiors
... sudden speed-up in the rotation speed of the neutron star, which relaxes afterwards to the slow declining trend. These glitches, alongside the temperature data from the observed radiation, provide significant clues for study of the physics of pulsars. On the theoretical side, Thomas Gold, and later ...
... sudden speed-up in the rotation speed of the neutron star, which relaxes afterwards to the slow declining trend. These glitches, alongside the temperature data from the observed radiation, provide significant clues for study of the physics of pulsars. On the theoretical side, Thomas Gold, and later ...
Matter: properties and characteristics COPY
... a. mass b. shell c. compound d. element 36. Which of the following is NOT found in the nucleus? a. Proton b. Neutron c. Electron 37. The atomic of an atom is based on the number of protons in the nucleus. a. mass b. shell c. group d. number 38. Two or more different ato ...
... a. mass b. shell c. compound d. element 36. Which of the following is NOT found in the nucleus? a. Proton b. Neutron c. Electron 37. The atomic of an atom is based on the number of protons in the nucleus. a. mass b. shell c. group d. number 38. Two or more different ato ...
Chemistry - nyostrander.us
... 3. State the model that first included electrons as subatomic particles. [2] The Thomson model first included electrons (the negatively charged particles). _________________________________________________________________________ 4. State one conclusion about the internal structure of the atom that ...
... 3. State the model that first included electrons as subatomic particles. [2] The Thomson model first included electrons (the negatively charged particles). _________________________________________________________________________ 4. State one conclusion about the internal structure of the atom that ...
Neutron stars and quark stars - Goethe
... Signals for Strange Stars? similar masses and radii, cooling, surface (crust), . . . but look for • extremely small mass, small radius stars (includes strangelets!) • strange dwarfs: small and light white dwarfs with a strange star core (Glendenning, Kettner, Weber, 1995) • super-Eddington luminosi ...
... Signals for Strange Stars? similar masses and radii, cooling, surface (crust), . . . but look for • extremely small mass, small radius stars (includes strangelets!) • strange dwarfs: small and light white dwarfs with a strange star core (Glendenning, Kettner, Weber, 1995) • super-Eddington luminosi ...